扩展功能
文章信息
- 俞冬升, 蔡大敏, 陈婕妤, 吕方怡, 花扣珍, 银国利, 王俊娟
- YU Dongsheng, CAI Damin, CHEN Jieyu, LYU Fangyi, HUA Kouzhen, YIN Guolin, WANG Junjuan
- 纤维型肌动蛋白对间充质干细胞衰老的调节作用及其机制
- Regulation effect of F-actin on senescence of mesenchymal stem cells and its mechanism
- 吉林大学学报(医学版), 2018, 44(03): 483-486
- Journal of Jilin University (Medicine Edition), 2018, 44(03): 483-486
- 10.13481/j.1671-587x.20180305
-
文章历史
- 收稿日期: 2017-12-13
2. 杭州医学院解剖学与组织胚胎学教研室, 浙江 杭州 310014
2. Department of Anatomy and Histology and Embryology, Hangzhou Medical College, Hangzhou 310014, China
间充质干细胞(mesenchymal stem cells,MSCs)是一类具有自我更新、复制和多向分化潜能的细胞[1-2]。多项动物研究和早期临床试验[3-5]证明:异体MSCs移植可用于治疗多种疾病。MSCs移植需要大量细胞,需要对分离的MSCs在移植前进行体外扩增[6]。随着MSCs体外培养传代次数的增加,其分化潜能和增殖能力逐渐降低,细胞呈现衰老的特征[7],成为限制临床应用的严重瓶颈。细胞形态的改变是细胞衰老的重要特征[8]。通常衰老的细胞体积增大,并呈现扁平状。有研究[9-10]比较年老和年轻供者来源的骨髓MSCs特征显示:与年轻供者比较,年老供者的MSCs虽具一定的增殖和分化能力,但细胞骨架流动性和重塑性降低,导致了细胞结合、物质转运和物质代谢等功能明显降低,同时细胞对外界理化环境刺激的反应迟缓,从而在功能上影响了MSCs在组织再生修复中的作用。然而细胞骨架影响MSCs衰老的具体机制尚未见相关报道。
细胞骨架由微丝、微管和中间纤维3种结构构成,其中微丝由纤维型肌动蛋白(fibrous actin,F-actin)聚合而成[11],细胞骨架蛋白F-actin的聚合可以影响细胞的迁移、增殖和分化等能力[12-15]。本研究采用人骨髓间充质干细胞(human bone marrow mesenchymal stem cells,hBMSCs)体外复制性衰老模型,探讨F-actin及其下游分子YAP在hBMSCs复制性衰老中的变化,使用F-actin抑制剂处理hBMSCs,检测hBMSCs中衰老相关指标的改变,初步阐明其相关分子机制。
1 材料与方法 1.1 细胞、主要试剂和仪器本实验所用细胞为hBMSCs,取自车祸患者截肢骨内的骨髓,已获得杭州医学院伦理委员会批准。DMEM培养基和胎牛培养基(美国Gibco公司),青霉素、链霉素、胰蛋白酶、牛血清白蛋白(BSA)、PBS、4%多聚甲醛、L-谷氨酰胺、鬼丙环肽Phalloidin和Actin-stain 555 Phalloidin(美国Cytoskeleton公司),F-actin抑制剂Latrunculin B(美国Abcam公司),YAP一抗和Ki67一抗(上海Santa Cruz公司),SA-β-Gal染液、成骨诱导液、成软骨诱导液和成脂诱导液(美国Oricell cyagen公司),488二抗(美国Abbkine公司)。生物安全柜(上海博迅医疗生物仪器股份有限公司),超净台(苏州医疗器械七厂),80℃超低温冰箱(美国Thermo公司),冰冻切片机(德国Microm公司),水浴锅(美国Millipore公司),PCR仪(美国Thermo Fisher公司),灭菌锅(上海博迅医疗生物仪器股份有限公司),CO2培养箱和纯水仪(美国Millipore公司),烘箱和离心机(德国Eppendoff公司),倒置荧光电子显微镜(日本Olympus公司),水浴锅(上海医疗器械七厂),高速离心机(德国Eppendoff公司),电泳仪(北京六一生物科技有限公司),Hitachi S-3000扫描电子显微镜(日本Hitachi公司)等。
1.2 hBMSCs的分离和培养取车祸患者截肢骨中的骨髓,将取得的骨髓置于无菌肝素管中离心去上清,采用PBS液调整骨髓细胞浓度至1×106 mL-1,按体积比1:1分别加入Ficoll分离液和骨髓细胞悬液,1 800 r·min-1离心20 min,吸取界面单个核细胞,采用PBS洗涤2次,加入细胞培养液后以1×106cm-1的密度接种于25 cm2培养瓶中,置于含5%CO2、37℃孵箱中培养。
1.3 hBMSCs三系分化能力的检测采用胰酶消化hBMSCs(37℃、2 min)后,采用含血清培养基终止胰酶作用,吹打离心(×200 g、5 min)去上清。将细胞重悬于含10%胎牛血清的L-DMEM培养基后进行细胞计数。分别接种至相应孔板中,在成骨诱导液中诱导培养2周,成脂诱导液中诱导培养3周,成软骨诱导液中诱导培养5周,诱导结束后弃去诱导液。分别用茜素红染色、SO染色和油红O染色确定诱导效果。
1.4 细胞分组原代hBMSCs长满后按1:3传代,在含5%CO2、37℃孵箱中培养。细胞长至80%左右时将细胞分组,分为对照组(P2代hBMSCs,不进行任何处理)、F-actin抑制剂组(2.5 μmol·L-1 F-actin抑制剂Latrunculin B处理P2代hBMSCs1 h)和P11代hBMSCs组(P2代hBMSCs连续传代得到P11代hBMSCs)。
1.5 免疫荧光染色观察各组hBMSCs中Ki67阳性细胞、F-actin的形态和聚合情况及YAP在细胞内的亚细胞定位待各组细胞分别长满至约60%,吸去培养基,PBS清洗3次,每次5 min。4%多聚甲醛溶液固定15 min后,PBS洗去多余固定液。采用0.2%TritonX-100透膜5 min,采用PBS清洗3次,每次5 min。用5% BSA室温封闭30 min,用1%BSA稀释一抗(YAP、Ki67和Acti-stain 555 Phalloidin),加入稀释好的一抗,置于湿盒里孵育,4℃过夜。次日,采用PBS洗涤3次,每次5 min。在孵育了YAP、Ki67一抗的样本中加入1%BSA稀释的488二抗(山羊抗鼠)避光孵育1 h后,采用PBS洗涤3次。加入DAPI进行细胞核染色5 min,采用PBS洗涤3次,每次5 min。采用免疫荧光封片剂封片,暗盒保存。在激光共聚焦显微镜下观察hBMSCs中Ki67阳性细胞、F-actin的形态和聚合情况及YAP在细胞内的亚细胞定位。
1.6 SA-β-Gal染色检测各组hBMSCs中SA-β-Gal染色阳性细胞数待各组hBMSCs分别长至约70%,吸去培养基,采用PBS洗涤细胞2次,加入1 mL SA-β-Gal染色固定液固定15 min。PBS洗涤3次,每次5 min,每孔加入1 mL染色工作液,37℃孵育过夜。普通光学显微镜下观察显示细胞衰老的SA-β-Gal染色阳性细胞数。
2 结果 2.1 hBMSCs的成骨、成脂和成软骨分化能力成功分离并培养得到hBMSCs,人骨髓中分离得到的hBMSCs为类成纤维细胞(图 1A,见插页一),细胞融合时呈现螺旋旋涡样生长。细胞分别在成骨、成脂和成软骨诱导液中诱导后采用茜素红染色、油红O染色和SO染色检测诱导效果。成骨分化诱导后茜素红染色阳性(图 1B,见插页一),成脂肪分化诱导后油红O染色阳性(图 1C,见插页一),成软骨分化诱导后SO染色阳性(图 1D,见插页一),证明分离得到的hBMSCs具有成骨、成脂和成软骨分化的能力。

|
| A: hBMSCs under light microscope(Bar = 200 μm); B: Osteogenic differentiation ability, Alizarin red staining(Bar = 50μm); C:Adipogenic differentiation ability, Oil red staining(Bar =100 μm); D:Chondrogenic differentiation ability, SO staining (Bar = 50 μm). 图 1 hBMSCs的成骨、成脂和成软骨分化能力 Figure 1 Osteogenic adipogenic and chondrogenic differentiation abilities of hBMSCs(seen on page 485in paragraph) |
|
|
通过Ki67荧光染色标记各组hBMSCs中处于增殖周期中的细胞,通过SA-β-Gal染色标记各组细胞中衰老的细胞。与对照组比较,P11代hBMSCs组SA-β-Gal染色阳性细胞数明显增多,而Ki67阳性细胞数明显减少。见图 2(插页二)。

|
| 图 2 各组hBMSCs在传代过程中细胞衰老的鉴定(Bar = 100 μm) Figure 2 Identification of cell senescence during passage of hBMSCs in various groups(Bar = 100 μm)(seen on page 485in paragraph) |
|
|
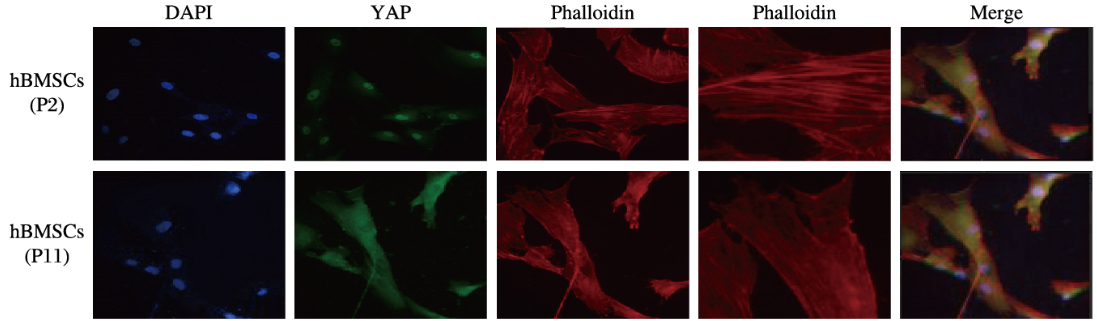
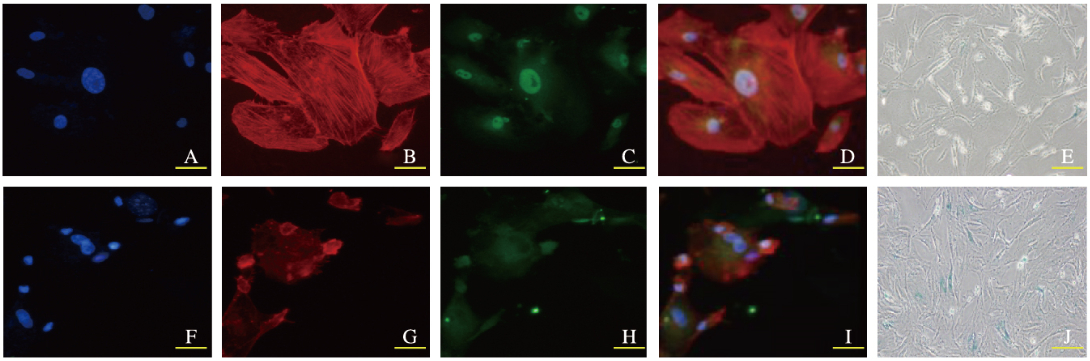
对照组hBMSCs中的微丝纤维束较多较粗,F-actin长度较长,YAP主要集中在细胞核(YAP在细胞核内为活化态);P11代hBMSCs组细胞中的微丝纤维束明显减少,F-actin长度变短,YAP主要集中在胞浆(YAP在胞浆为失活态);同时hBMSCs中YAP出核的细胞SA-β-Gal染色阳性;F-actin抑制剂组F-actin聚合异常,YAP出核失活,SA-β-Gal染色阳性细胞数明显增多。见图 3~5(插页二)。
|
| 图 3 免疫荧光染色检测各组hBMSCs中YAP分布和F-actin形态表现(Bar=50 μm) Figure 3 YAP distribution and morphology of F-actin in hBMSCs in various groups detected by immunofluorescence staining(Bar = 50 μm)(seen on page 485in paragraph) |
|
|

|
| 图 4 荧光染色和SA-β-Gal染色共定位检测P2和P11 hBMSCs中YAP分布(Bar=50 μm) Figure 4 YAP distribution in P2 and P11 hBMSCs detected by fluorescence staining and SA -β- Gal dyeing positioning (Bar = 50 μm)(seen on page 485in paragraph) |
|
|
|
| A-E:Control group(Bar=50 μm); F-I:F-actin inhibitor group(Bar=50 μm); J: F-actin inhibitor group (Bar=100 μm); A, F:DAPI fluorescence staining; B, G:Phalloidin fluorescence staining; C, H:YAP fluorescence staining; D, I:Merge; E, J: SA-β-Gal staining. 图 5 荧光染色检测各组hBMSCs衰老形态表现 Figure 5 Morphology of senescence of hBMSCs in various groups detected by fluorescence staining(seen on page 485in paragraph) |
|
|
干细胞衰老影响体外培养的MSCs数量和质量,传代次数较多的MSCs临床疗效明显不如传代次数较少的MSCs,使得MSCs衰老成为其临床应用的制约因素[16]。研究[17]表明:细胞衰老是老年性疾病的关键致病因子,因此研究MSCs的衰老及相关分子机制具有重要意义。研究[8]显示:MSCs在传代过程中伴随细胞形态表现的改变,而细胞骨架蛋白决定了细胞的形态结构。本研究结果表明:随着细胞代数的增加,细胞表面积明显增大,微丝纤维束减少,F-actin长度变短、变细且聚合减少。
YAP作为一种多功能转录共激活因子,在MSCs周期进程中起关键作用[18]。同时,YAP也被认为参与了细胞的衰老过程,YAP表达随着细胞传代次数的增加而逐渐降低,通过敲除YAP可直接诱导人胚肺成纤维细胞和肝脏卫星细胞的衰老[19-20]。本研究结果显示:YAP在不同代数hBMSCs中的亚细胞定位呈现明显差异,且YAP出核的细胞SA-β-Gal染色阳性。
YAP的生物活性可受F-actin调控,细胞中F-actin的聚合差异可以导致YAP的细胞定位不同,从而影响细胞的迁移、增殖或分化[11-14, 21]。因此本文作者探讨了F-actin是否通过调节YAP活性影响hBMSCs的衰老。本研究结果显示:F-actin抑制剂可以促进YAP出核失活,同时明显增加SA-β-Gal染色阳性的细胞。
综上所述,F-actin和YAP参与了hBMSCs的衰老过程,F-actin抑制剂可以抑制F-actin聚合,进一步调节YAP的出入核从而影响hBMSCs的衰老。本研究结果从分子生物学角度为MSCs的衰老提供了新的认知,MSCs具有广阔的临床应用前景。
| [1] | Baldari S, Di GR, Piccoli M, et al. Challenges and strategies for improving the regenerative effects of mesenchymal stromal cell-based therapies[J]. Int J Mol Sci, 2017, 18(10): 2087. DOI:10.3390/ijms18102087 |
| [2] | Kim HJ, Park JS. Usage of human mesenchymal stem cells in cell-based therapy:advantages and disadvantages[J]. Dev Reprod, 2017, 21(1): 1–10. DOI:10.12717/DR.2017.21.1.001 |
| [3] | Afizah H, Hui JH. Mesenchymal stem cell therapy for osteoarthritis[J]. J Clin Orthop Trauma, 2016, 7(3): 177–182. DOI:10.1016/j.jcot.2016.06.006 |
| [4] | de Windt TS, Vonk LA, Slaper-Cortenbach IC, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons[J]. Stem Cells, 2017, 35(1): 256–264. DOI:10.1002/stem.2475 |
| [5] | Liu S, Liu D, Chen C, et al. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus[J]. Cell Metab, 2015, 22(4): 606–618. DOI:10.1016/j.cmet.2015.08.018 |
| [6] | Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells[J]. Science, 1999, 284(5411): 143–147. DOI:10.1126/science.284.5411.143 |
| [7] | Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells:functional changes and implications in stem cell-based therapy[J]. Int J Mol Sci, 2016, 17(7): 1164. DOI:10.3390/ijms17071164 |
| [8] | Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells:a continuous and organized process[J]. PLoS One, 2008, 3(5): e2213. DOI:10.1371/journal.pone.0002213 |
| [9] | Kasper G, Mao L, Geissler S, et al. Insights into mesenchymal stem cell aging:involvement of antioxidant defense and actin cytoskeleton[J]. Stem Cells, 2009, 27(6): 1288–1297. DOI:10.1002/stem.v27:6 |
| [10] | Kretlow JD, Jin YQ, Liu W, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells[J]. BMC Cell Biol, 2008, 9(1): 60. DOI:10.1186/1471-2121-9-60 |
| [11] | Kim S, Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation[J]. Nat Rev Mol Cell Biol, 2010, 11(1): 75–81. DOI:10.1038/nrm2818 |
| [12] | Wada K, Itoga K, Okano T, et al. Hippo pathway regulation by cell morphology and stress fibers[J]. Development, 2011, 138(18): 3907–3914. DOI:10.1242/dev.070987 |
| [13] | Liu F, Lagares D, Choi KM, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis[J]. Am J Physiol Lung Cell Mol Physiol, 2015, 308(4): L344–357. DOI:10.1152/ajplung.00300.2014 |
| [14] | Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors[J]. Cell, 2013, 154(5): 1047–1059. DOI:10.1016/j.cell.2013.07.042 |
| [15] | Fernández BG, Gaspar P, Brás-Pereira C, et al. Actin-capping protein and the hippo pathway regulate F-actin and tissue growth in Drosophila[J]. Development, 2011, 138(11): 2337–2346. DOI:10.1242/dev.063545 |
| [16] | Baker N, Boyette LB, Tuan RS. Characterization of bone marrow-derived mesenchymal stem cells in aging[J]. Bone, 2015, 70: 37–47. DOI:10.1016/j.bone.2014.10.014 |
| [17] | Childs BG, Durik M, Baker DJ, et al. Cellular senescence in aging and age-related disease:from mechanisms to therapy[J]. Nat Med, 2015, 21(12): 1424–1435. DOI:10.1038/nm.4000 |
| [18] | Caliari SR, Vega SL, Kwon M, et al. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments[J]. Biomaterials, 2016, 103: 314–323. DOI:10.1016/j.biomaterials.2016.06.061 |
| [19] | Xie Q, Chen J, Feng H, et al. YAP/TEAD-mediated transcription controls cellular senescence[J]. Cancer Res, 2013, 73(12): 3615–3624. DOI:10.1158/0008-5472.CAN-12-3793 |
| [20] | Jin H, Lian N, Zhang F, et al. Inhibition of YAP signaling contributes to senescence of hepatic stellate cells induced by tetramethylpyrazine[J]. Eur J Pharm Sci, 2016, 96: 323–333. |
| [21] | 刘源, 王海萍, 吕洋, 等. 不同浓度Wnt-11诱导骨髓间充质干细胞向心肌样细胞定向分化的实验研究[J]. 西安交通大学学报:医学版, 2017, 38(2): 199–205. |
 2018, Vol. 44
2018, Vol. 44


