扩展功能
文章信息
- 于云鹤, 杜烨, 韩冰, 李嗣杰, 宋乐连, 范志民
- YU Yunhe, DU Ye, HAN Bing, LI Sijie, SONG Lelian, FAN Zhimin
- 新辅助化疗对Luminal B型乳腺癌各亚组的疗效观察及预后分析
- Observation on curative effects of neoadjuvant chemotherapy for different subtypes of Luminal B breast cancer and analysis on its prognosis
- 吉林大学学报(医学版), 2018, 44(02): 356-362
- Journal of Jilin University (Medicine Edition), 2018, 44(02): 356-362
- 10.13481/j.1671-587x.20180227
-
文章历史
- 收稿日期: 2017-09-18
乳腺癌是一类高度异质性的恶性肿瘤,即使是同一种分子分型在临床表现及治疗反应上均存在较大的差异。目前被公认的乳腺癌分子分型主要有4种:Luminal A型、Luminal B型、单纯Her-2过表达型及三阴型, 其中最常见的乳腺癌分子分型是Luminal B型,根据2013年St.Gallen会议中的报道,Luminal B型乳腺癌占全部乳腺癌的近40%[1]。Luminal B型乳腺癌具有孕激素受体(progesterone receptor, PR)阴性或低表达、人表皮生长因子2(human epidermal growth factor-2, Her-2)阳性、细胞增殖核抗原(Ki-67)高表达的特点,实际上是一类异质性群体,故可将其分为具有不同特点的亚组。新辅助化疗(neoadjuvant chemotherapy,NAC)已成为局部晚期乳腺癌的标准治疗,NAC后获得病理完全缓解(pathological complete response, pCR)是远期生存获益的一个重要的预后因素。目前国外最新研究[2]显示:行NAC的Luminal B型乳腺癌患者无病生存率(disease-free survival, DFS)明显改善,与Her-2或PR状态无关。而国内文献[3]指出:行TAC方案NAC的Luminal B型乳腺癌患者,根据Her-2及Ki-67表达状态分成的不同亚组间DFS比较差异有统计学意义。为进一步探究Luminal B型乳腺癌亚组的临床特征及预后特点,本研究将Luminal B型乳腺癌分成具有不同特点的亚组,观察各亚组在NAC中的疗效反应和预后差异。
1 资料与方法 1.1 一般资料选择2011年1月—2015年12月本院乳腺外科收治并完成预计NAC疗程的原发性乳腺浸润性癌患者450例,根据分子分型标准[4],Luminal A型乳腺癌34例,Luminal B型乳腺癌255例,单纯Her-2过表达型乳腺癌75例,三阴型乳腺癌86例。其中Luminal B型乳腺癌255例,占同期NAC患者56.9%,排除FISH检测结果为临界值的患者9例,本研究共纳入246例Luminal B型乳腺癌患者。患者均为女性,年龄、绝经状态、家族史及治疗方式等临床资料完整。就诊前均未接受化疗,内分泌治疗或局部放疗,ECOG评分均为1分。NAC前常规行肺部CT、腹部彩超或腹部CT、全身骨扫描等检查,均无明确的远处转移,第1次化疗前均行超声引导下空心针穿刺活检术,明确病理为浸润性癌,同时采用免疫组织化学法检测癌组织中雌激素受体(estrogen receptor, ER)、PR、Her-2及Ki-67的表达情况,其中Her-2结果为++的患者行FISH检测,依据检测结果分为扩增、不扩增及临界值。参照NCCN指南选择最佳化疗方案,化疗方案包括:CMF(环磷酰胺,甲氨蝶呤,氟尿嘧啶)、TAC(环磷酰胺,吡柔比星,多西他赛/紫杉醇)、TCH(多西他赛/紫杉醇,卡铂,曲妥珠单抗)、AC-T(环磷酰胺,吡柔比星,序贯多西他赛/紫杉醇)和AC-TH(环磷酰胺,吡柔比星,序贯多西他赛/紫杉醇联合曲妥珠单抗)等常规化疗方案,未完成预计化疗疗程的患者不纳入此研究。
完成NAC后行手术治疗,术后参照NCCN指南给予患者术后治疗方案的建议。主要观察指标为临床疗效反应评价、病理疗效反应评价、是否达到pCR及DFS。
1.2 分型和分组标准参照2013年St.Gallen会议专家共识[4],根据免疫组织化学指标将Luminal B型乳腺癌分为Her-2阴性和Her-2阳性。①Her-2阴性。ER阳性和Her-2阴性,且至少符合以下一项:a.Ki-67高表达;b.PR阴性或低表达;c.多基因表达示高复发风险。②Her-2阳性。ER阳性,Her-2过表达或扩增,Ki-67和PR任何水平。Ki-67以14%作为高低表达的界值[5],PR以20%作为高低表达的界值[6]。
本研究根据PR、Her-2及Ki-67的表达将Luminal B型乳腺癌分成3个亚组:A亚组(PR低表达组),Her-2阴性且PR阴性或<20%,Ki-67任何水平;B亚组(PR高表达组),Her-2阴性,PR≥20%且Ki-67≥14%;C亚组(Her-2阳性组),Her-2阳性,Ki-67和PR任何水平。
1.3 疗效判定标准 1.3.1 临床疗效评价标准根据2017年中国抗癌协会乳腺癌诊治指南与规范[7]推荐,临床疗效评价按国际实体瘤疗效(RECIST1.1)评判标准[8]:①完全缓解(complete response, CR),临床检查肿瘤完全消失;②部分缓解(partial response, PR),肿瘤最大直径或最大径之和(多个靶病灶)减少≥30%;③疾病稳定(stable disease, SD),不符合PR及疾病进展(progressive disease,PD)标准者;④PD,肿瘤最大直径或最大径之和(多个靶病灶)增大≥20%, 病灶增大径>5mm或出现新病灶。每周期化疗前均评估原发肿瘤大小变化,计算临床疗效,有效(PR、CR)则继续原化疗方案,若无效(SD、PD),则考虑换方案或条件允许行手术治疗。
1.3.2 术后病理评价标准按Miller and Payne系统[9]将化疗前的空心针活检标本与化疗后的手术标本进行比较,主要针对NAC后残余肿瘤的细胞丰富程度进行评估,共分为5级。其中1级(G1)为浸润癌细胞无改变或仅个别癌细胞发生改变,癌细胞数量总体未减少;2级(G2)为浸润癌细胞轻度减少,但总数量仍高,癌细胞减少不超过30%;3级(G3)为浸润癌细胞减少介于30%~90%;4级(G4)为浸润癌细胞显著减少超过90%,仅残存散在的小簇状癌细胞或单个癌细胞;5级(G5)为原肿瘤瘤床部位已无浸润癌细胞,但可存在导管原位癌。病理完全缓解(pathological complete response,pCR):NAC原发肿瘤区域及区域淋巴结均无浸润性癌残留或仅有导管癌原位残留。Miller and Payne系统中1~2级视为无效,3~5级视为有效,其中若原发肿瘤达到5级及区域淋巴结均无浸润性癌残留或仅有导管癌原位残留属pCR。
1.4 统计学分析应用SPSS22.0统计软件进行统计学分析。采用卡方检验对计数资料进行比较,少于5例的检验用Fisher exact test方法。生存分析采用Log-rank检验。以P<0.05为差异有统计学意义。
2 结果 2.1 4种分子分型乳腺癌患者的一般临床特征及疗效反应根据分子分型标准[2],Luminal A型乳腺癌34例,Luminal B型乳腺癌255例,单纯Her-2过表达型乳腺癌75例,三阴型乳腺癌86例。4种分型患者年龄、原发肿瘤大小、淋巴结状态和肿瘤TNM分期比较差异均无统计学意义(P>0.05)。见表 1。Luminal A型、Luminal B型、Her-2过表达型及三阴型患者的临床有效率分别为61.7%、59.2%、77.3%和78.6%,差异有统计学意义(P < 0.01)。450例患者中有70例达到pCR,pCR率为15.6%,Luminal A型、Luminal B型、单纯Her-2过表达型及三阴型患者pCR率分别为0%、7.8%、24.0%和37.0%,差异有统计学意义(P < 0.01)。4种分型患者病理疗效比较差异无统计学意义(P>0.05)。见表 1。
| Clinical characteristic | Luminal A (n=34) |
Luminal B (n=255) |
Her-2 over-expression (n=75) | Triple negative (n=86) |
χ2 | P |
| Age(year) | ||||||
| ≥35 36-59 ≤60 |
3 24 7 |
16 207 32 |
6 59 10 |
5 67 14 |
2.834 | 0.829 |
| Tumor size(T) | ||||||
| T1 T2 T3 T4 |
3 25 4 2 |
31 182 24 18 |
13 47 8 7 |
8 61 12 5 |
5.325 | 0.805 |
| Lymph node status | ||||||
| N0 N1 N2 N3 |
16 17 1 0 |
98 135 22 0 |
25 42 8 0 |
30 42 12 2 |
13.923 | 0.123 |
| TNM staging | ||||||
| Ⅰ Ⅱ Ⅲ |
1 29 4 |
11 201 43 |
4 53 18 |
1 60 25 |
10.206 | 0.116 |
| Clinical response | ||||||
| Effective(CR+PR) Invalid(SD+PD) Effective rate |
18 16 61.7% |
151 104 59.2% |
58 17 77.3% |
70 16 78.6% |
21.191 | 0.000 |
| Pathological response | ||||||
| Effective(G3-G5) Invalid(G1-G2) Effective rate |
21 13 61.7% |
185 70 72.5% |
63 12 74.1% |
61 25 70.9% |
7.025 | 0.071 |
| pCR | ||||||
| pCR Not pCR Rate of pCR |
0 34 0% |
20 235 7.8% |
18 57 24.0% |
32 54 37.0% |
52.579 | 0.000 |
本研究中Luminal B型3个亚组患者共246例,其中A亚组58例、B亚组124例和C亚组64例。年龄28~68岁,中位年龄49岁,中位随访时间39.7个月(3.5~74.0个月)。3个亚组患者淋巴结转移率比较差异有统计学意义(P < 0.05),3个亚组中A亚组患者淋巴结转移率最高。3个亚组患者年龄、原发肿瘤大小和肿瘤TNM分期比较差异均无统计学意义(P>0.05)。见表 2。246例中有21例患者达到pCR,为8.5%,各亚组患者pCR率比较差异有统计学意义(P < 0.01)。C亚组pCR率最高,B亚组pCR率最低。各亚组临床疗效和病理疗效比较差异均无统计学意义(P>0.05)。见表 2。
| Clinical characteristic | PR low expression group (n= 58) |
PR high expression group (n=124) |
Her-2 positive group (n=64) |
χ2 | P |
| Ages(year) | |||||
| ≥35 36-59 ≤60 |
3 43 12 |
7 106 11 |
4 53 7 |
5.326 | 0.255 |
| Tumor size(T) | |||||
| T1 T2 T3 T4 |
6 41 7 4 |
18 89 9 8 |
7 47 6 4 |
1.830 | 0.935 |
| Lymph node status | |||||
| N0 N1 N2 |
18 29 11 |
50 69 5 |
27 32 5 |
11.915 | 0.018 |
| TNM staging | |||||
| Ⅰ Ⅱ Ⅲ |
1 44 13 |
8 99 17 |
2 52 10 |
4.310 | 0.366 |
| Clinical response | |||||
| Effective(CR+PR) Invalid(SD+PD) Effective rate |
34 24 58.6 |
66 58 53.2 |
44 20 68.7 |
4.192 | 0.123 |
| Pathological response | |||||
| Effective(G3-G5) Invalid(G1-G2) Effective rate |
41 17 70.7% |
85 39 68.5% |
54 10 84.3% |
5.624 | 0.060 |
| pCR | |||||
| pCR Not pCR Rate of pCR |
7 51 12.0% |
4 120 3.2% |
10 54 15.6% |
9.525 | 0.009 |
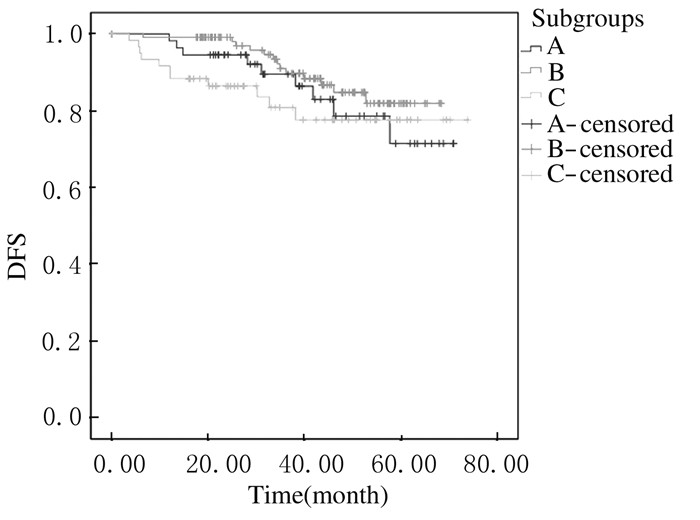
3个亚组生存曲线的Log-Rank检验差异无统计学意义(P>0.05)。见图 1。但3个亚组患者3年DFS与5年DFS略有差异,B亚组患者3年DFS和5年DFS均稍高于其他2组。见表 3。本研究随访了同时期的75例Her-2过表达型乳腺癌患者(图 2中H组)。Luminal B型乳腺癌中Her-2阳性组(C亚组)与单纯Her-2过表达型乳腺癌患者DFS比较差异有统计学意义(Log-rank检验P < 0.05),C亚组的DFS较高。见图 2。
|
| A:PR low expression group; B:PR high expression group; C:Her-2 positive group. 图 1 Luminal B型乳腺癌3个亚组患者生存分析 Figure 1 Survival analysis of Luminal B breast cancer patients in three subgroups |
|
|
| (η/%) | ||
| Subgroup | 3-year DFS | 5-year DFS |
| PR low expression | 89.5 | 71.4 |
| PR high expression | 89.7 | 81.9 |
| Her-2 positive | 80.0 | 77.6 |

|
| C:Her-2 positive group; H:Her-2 overexpression group. 图 2 Luminal B型乳腺癌患者中Her-2阳性组与单纯Her-2过表达型组生存分析 Figure 2 Survival analysis of Her-2 positive subgroup and Her-2 overexpression group in Luminal B breast cancer patients |
|
|
本研究中4种分子分型乳腺癌患者NAC后的pCR率比较差异有统计学意义,其中激素受体阳性的Luminal A型及Luminal B型pCR率较低,而单纯Her-2过表达型及三阴型pCR率较高;在LuminalB型乳腺癌亚组分析中3个亚组pCR率分别为12.0%、3.2%和15.6%,Her-2阳性组患者更易达到pCR,差异有统计学意义,此结果与Bonnefoi等[10]研究结果一致。在EORTC10994/BIG1-100临床试验研究中,不同分子亚型乳腺癌患者NAC的疗效反应不同,激素受体阳性患者NAC后很难获得pCR,而Her-2阳性和三阴型患者行NAC可获得更高的pCR率。另有研究[11]显示:对于三阴型、Her-2阳性及Lunimal型且组织学分型3级的乳腺癌患者,NAC后达到pCR的患者具有较好的预后,而对于侵袭性较低的Luminal A型患者,NAC后达到pCR并不改善患者预后。但Luminal A型乳腺癌患者化疗敏感性差,内分泌治疗敏感性高,内分泌治疗已成为Luminal A型乳腺癌的标准治疗之一。而Luminal B型乳腺癌与Luminal A型乳腺癌相比对内分泌相对耐药,而化疗不如单纯Her-2过表达型及三阴型乳腺癌敏感,较难达到pCR,是一种具有临床难治性特点的亚型。本研究根据PR、Her-2及Ki-67表达状态将Luminal B型乳腺癌进一步分成了具有不同特点的亚组,探讨各亚组在NAC中的疗效反应及预后差异,为Luminal B型乳腺癌的个体化治疗提供依据。
2013年St.Gallen会议专家共识[4]将PR状态加入到乳腺癌的分子分型中,对PR≤20%的Luminal型乳腺癌归入预后较差的Luminal B型中。本研究结果显示:PR低表达组(A组)的总体生存曲线与其他2组差异无统计学意义,证实将PR≤20%的Luminal型乳腺癌归入Luminal B型中有临床指导意义的。
有研究[6, 12-14]显示:PR在Luminal B乳腺癌中预后及内分泌治疗中具有重要意义。Yu等[14]研究发现PR阴性是Her-2阴性Luminal B型乳腺癌早期复发转移的高危因素。研究[16]显示:对于ER阳性、Her-2阴性、Ki67>14%的LuminalB型乳腺癌,PR>20%者的预后较PR<20%者更好。本研究结果显示:虽然3个亚组的总体生存曲线无显著性差异,但PR高表达组(B亚组)的3年及5年DFS均稍高于其他2组,即Luminal B型乳腺癌中PR高表达者有相对预后较好的趋势,故有理由相信PR高表达是Luminal B型乳腺癌预后较好的预测因素。
研究[15]显示:尽管ER和Her-2介导的信号通路在多个环节上相互交叉相互作用,但Her-2阳性的Luminal B型乳腺癌仍可以在内分泌治疗中获益。本研究中Luminal B型乳腺癌中Her-2阳性组(C亚组)与同时期单纯Her-2过表达型乳腺癌组(H组)的生存曲线差异有统计学意义,即Her-2阳性Luminal B型乳腺癌的预后优于单纯Her-2过表达型乳腺癌。晚期乳腺癌的回顾性分析[16-17]显示:对于Her-2阳性LuminalB型乳腺癌,在化疗及靶向治疗后继续给予内分泌治疗能够明显改善患者无进展生存时间。总之,Her-2阳性Luminal B型乳腺癌患者在一线治疗后进行内分泌治疗能够改善预后[18]。且有研究[19]显示:通过使用曲妥珠单抗降低Her-2受体的活性,可恢复Her-2阳性Luminal B型乳腺癌中激素治疗的反应。因此,对于局部晚期的Her-2阳性Luminal B型乳腺癌推荐化疗、靶向治疗、内分泌治疗及手术等综合性治疗。
本研究结果显示:局部晚期的Luminal B型乳腺癌患者的治疗应根据不同亚组的特点选择更为获益的治疗方案。根据NCCN指南[20]及国内指南[7],对于局部晚期的Her-2阳性Luminal B型乳腺癌治疗,均推荐包含曲妥珠单抗的化疗方案,结合本研究结果,其术后辅助内分泌治疗也必不可少。对于PR高表达的Luminal B型乳腺癌化疗敏感性相对较差,而内分泌治疗获益较大,这类患者的内分泌治疗应得到更多的重视。对于PR低表达的Luminal B型乳腺癌患者,5年DFS最低,这可能意味着这类患者的远期生存较差。研究[21]显示:NAC后未达到pCR的Her-2阴性乳腺癌患者,继续口服卡培他滨治疗组的5年DFS要高于未口服卡培他滨治疗组,卡培他滨治疗的安全性也基本令人满意。在中国卡培他滨多用于转移性乳腺癌的治疗[22],因此对于NAC后未达到pCR的PR低表达Luminal B型乳腺癌患者,是否可以口服卡培他滨治疗或行其他方案化疗,有待进一步研究。
综上所述,Luminal B型乳腺癌可根据PR、Her-2及Ki-67状态分为3个不同的亚组,NAC后病理疗效分析显示:Her-2阳性型Luminal B型乳腺癌更易达到pCR;生存分析显示3个亚组的预后无显著性差异,但PR高表达者有相对预后较好的趋势。本研究存在病例数较少、预后随访时间较短等局限性,有必要进行大样本量前瞻性研究对3种同亚组的特点进一步探究,为临床诊治过程中提供获益最大的综合治疗方案提供依据。
| [1] | Metzger-Filho O, Sun Z, Viale G, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease:results from international breast cancer study group trials Ⅷ and Ⅸ[J]. J Clin Oncol, 2013, 31: 3083–3090. DOI:10.1200/JCO.2012.46.1574 |
| [2] | Zhang J, Laubacher JS, Ramirez MT, et al. The role of adjuvant chemotherapy in Luminal B breast cancer[J]. J Clin Oncol, 2014, 32(26-suppl): 156. |
| [3] | 杨福兰, 倪军, 黄兴伟, 等. TAC对luminal B型乳腺癌的新辅助化疗疗效观察与预后分析[J]. 实用临床医学, 2016, 17(3): 32–35. |
| [4] | Goldhirsch A, Winer E, Coates A, et al. Personalizing the treatment of women with early breast cancer:highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013[J]. Ann Oncol, 2013, 24(9): 2206–2223. DOI:10.1093/annonc/mdt303 |
| [5] | Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes-dealing with the diversity of breast cancer:highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011[J]. Ann Oncol, 2011, 22(8): 1736–1747. DOI:10.1093/annonc/mdr304 |
| [6] | Prat A, Cheang MC, Martín M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined Luminal A breast cancer[J]. J Clin Oncol, 2013, 31(2): 203–209. DOI:10.1200/JCO.2012.43.4134 |
| [7] | 中国抗癌协会乳腺癌专业委员会. 中国抗癌协会乳腺癌诊治指南与规范(2017年版)[J]. 中国癌症杂志, 2017, 27(9): 695–760. |
| [8] | Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228–247. DOI:10.1016/j.ejca.2008.10.026 |
| [9] | Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy:prognostic significance and survival[J]. Breast, 2003, 12(5): 320–327. DOI:10.1016/S0960-9776(03)00106-1 |
| [10] | Bonnefoi H, Litière S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes:a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase Ⅲ trial[J]. Ann Oncol, 2014, 25(6): 1128–1136. DOI:10.1093/annonc/mdu118 |
| [11] | Cortazar P, Zhang L, Untch M, et al. Abstract S1-11:Meta-analysis results from the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC)[J]. Cancer Res, 2012, 72(24): S1–11. |
| [12] | Nishimukai A, Yagi T, Yanai A, et al. High Ki-67 expression and low progesterone receptor epression culd idependently lead to a worse prognosis for postmenopausal patients with estrogen receptor-positive and HER2-negative breast bancer[J]. Clin Breast Cancer, 2015, 15(3): 204–211. DOI:10.1016/j.clbc.2014.12.007 |
| [13] | Cancello G, Maisonneuve P, Rotmensz N, et al. Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse[J]. Ann Oncol, 2013, 24(3): 661–668. DOI:10.1093/annonc/mds430 |
| [14] | Yu Z, Li Z, Wu J, et al. progesterone receptor status and Ki-67 index may predict early relapse in Luminal B/HER2 negative breast cancer patients:A retrospective study[J]. PLoS One, 2014, 9(8): e95629. DOI:10.1371/journal.pone.0095629 |
| [15] | Hayashi N, Niikura N, Yamauchi H, et al. Adding hormonal therapy to chemotherapy and trastuzumab improves prognosis in patients with hormone receptor-positive and human epidermal growth factor receptor 2-positive primary breast cancer[J]. Breast Cancer Res Treat, 2013, 137(2): 523–531. DOI:10.1007/s10549-012-2336-6 |
| [16] | Saura C, Bendell J, Jerusalem G, et al. Phase Ib study of Buparlisib plus Trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on Trastuzumab-based therapy[J]. Clin Cancer Res, 2014, 20(7): 1935–1945. DOI:10.1158/1078-0432.CCR-13-1070 |
| [17] | Vaz-Luis I, Seah D, Olson EM, et al. Clinicopathological features among patients with advanced human epidermal growth factor-2-positive breast cancer with prolonged clinical benefit to first-line trastuzumab-based therapy:a retrospective cohort study[J]. Clin Breast Cancer, 2013, 13(4): 254–263. DOI:10.1016/j.clbc.2013.02.010 |
| [18] | Bonotto M, Gerratana L, Poletto E, et al. Measures of outcome in metastatic breast cancer:insights from a real-world scenario[J]. Oncologist, 2014, 19(6): 608–615. DOI:10.1634/theoncologist.2014-0002 |
| [19] | McGuire A, Kalinina O, Holian E, et al. Differential impact of hormone receptor status on survival and recurrence for HER2 receptor-positive breast cancers treated with Trastuzumab[J]. Breast Cancer Res Treat, 2017, 164(1): 221–229. DOI:10.1007/s10549-017-4225-5 |
| [20] | Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights:Breast Cancer, Version 1.2017[J]. J Natl Compr Cancer Netw, 2017, 15(4): 433–451. DOI:10.6004/jnccn.2017.0044 |
| [21] | Masuda N. adjuvant capecitabine for breast cancer after preoperative chemotherapy[J]. N Engl J Med, 2017, 376(22): 2147–2159. DOI:10.1056/NEJMoa1612645 |
| [22] | Chen X, Du F, Hong R, et al. Hormonal therapy might be a better choice as maintenance treatment than capecitabine after response to first-line capecitabine-based combination chemotherapy for patients with hormone receptor-positive and HER2-negative, metastatic breast cancer[J]. Cancer, 2016, 35(6): 46–52. |
 2018, Vol. 44
2018, Vol. 44


