扩展功能
文章信息
- 李胜男, 张海英, 孙千惠, 李艳茹, 葛鹤, 杜瑜, 王琳
- LI Shengnan, ZHANG Haiying, SUN Qianhui, LI Yanru, GE He, DU Yu, WANG Lin
- 水流动力学注射对小鼠肝损伤及肝脏的自然修复作用
- Liver injury induced by hydrodynamic injection and natural repair of liver in mice
- 吉林大学学报(医学版), 2018, 44(02): 275-280
- Journal of Jilin University (Medicine Edition), 2018, 44(02): 275-280
- 10.13481/j.1671-587x.20180213
-
文章历史
- 收稿日期: 2017-08-12
水流动力学注射(hydrodynamic injection)是将大体积(占小鼠体质量的8%~10%)质粒DNA溶液经小鼠尾静脉在5~8 s内快速注射入小鼠体内,进而获得转基因表达的方法,通常转基因在肝脏表达最多[1]。除DNA外,已成功应用此方法将RNA、蛋白甚至病毒等转移到动物体内,广泛应用于基因功能测试、基因治疗等研究以及病毒性肝炎模型的制备等[2-3]。由于静脉注射的大体积质粒DNA溶液迅速进入体循环,使心脏负荷过重,导致大量的液体淤积在肝脏,肝内压力升高,迫使肝窦内皮细胞窗增大,肝细胞膜出现暂时性小孔,质粒DNA进入肝细胞,进而表达目的基因[4]。此方法产生的压力将基因送入肝细胞的同时,也可能引起肝窦内皮细胞和肝细胞损伤。但是关于肝脏的形态学改变的研究[5]极少,其修复过程尚不清楚。本研究通过水流动力学注射法制备小鼠肝损伤模型,观察肝脏在损伤及修复过程中的形态学变化,检测与修复有关的因子,为更好地利用此方法进行科学研究提供依据,为进一步探讨水流动力学注射性肝损伤的修复机制奠定基础。
1 材料与方法 1.1 实验动物和肝损伤模型制备31只雌性Balb/c小鼠,体质量为18~20 g,购自吉林大学实验动物中心,动物许可证号:SCXK(吉)-2003-0001。用26 G针头将小鼠体质量10%的生理盐水(1.8~2.0 mL)在3 s内经尾静注射入小鼠体内。按照注射后时间点将小鼠分为5组:30 min组、8 h组、1 d组、3 d组和7 d组,每组5只。以6只未注射小鼠为对照组,记为0 min组。
1.2 各组小鼠血清丙氨酸氨基转移酶(alanine transaminase,ALT)测定注射生理盐水后,于不同时间点经内眦静脉取血,按照ALT试剂盒(上海科华-东菱诊断用品公司)说明检测血清ALT水平。
1.3 肝脏形态学观察分别于注射后不同时间点处死小鼠并称体质量。取肝脏观察大体改变,制备石蜡切片,经苏木精-伊红(HE)染色,显微镜下观察病理学改变。为了确定肝脏病理学改变的区域,根据参考文献[5]将围绕门静脉周围的肝细胞区域定为门静脉区,即1区;围绕在中央静脉周围的肝细胞区域定为中央静脉区,即3区;介于二者之间的肝细胞区域为2区。每只小鼠分别选取10个有代表性的区域,统计每高倍视野下的肝细胞数及双核细胞数。
1.4 免疫组织化学染色上述制备的石蜡切片,利用免疫组织化学染色(SP法)观察增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)的表达情况。一抗为鼠抗人PCNA单克隆抗体(福州迈新,1:300),二抗为羊抗鼠/兔IgG(福州迈新),DAB溶液显色,苏木精复染。以PBS代替一抗作为阴性对照。PCNA阳性表现为细胞核呈棕黄色。每只小鼠肝组织切片内分别选取10个视野,计算肝细胞及胆管细胞PCNA阳性细胞百分数,将其作为PCNA指数。
1.5 Real-time PCR法检测基因表达水平按照RNAiso Plus(日本TaKaRa公司)说明书提取肝组织总RNA;根据Reverse Transcriptase M-MLV(RNase H-)(日本TaKaRa公司)说明书进行逆转录反应(RT),按照FastStart Universal SYBR Green Master (ROX) (瑞士Roche公司)说明书进行Real-time PCR法检测,利用ABI 7300实时荧光定量PCR仪(美国ABI公司)检测各组小鼠肝组织中肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、白细胞介素6(interleukin-6,IL-6)、表皮生长因子(epidermal growth factor,EGF)、肝细胞生长因子(hepatocyte growth factor,HGF)、转化生长因子α(transforming growth factor-α,TGF-α)、Cyclin D1、血管内皮细胞生长因子(vascular endothelial growth factor,VEGF)、Bax和Bcl-2 mRNA表达水平,基因相对表达水平以2-ΔΔCt表示,其中ΔΔCt=(实验组Ct目的基因-实验组Ct内参基因) -(对照组Ct目的基因-对照组Ct内参基因)。以m-Arbp和β-actin为内参,引物设计见表 1。
| Gene | Primer sequence |
| TNF-α | F:5′-GAAACACAAGATGCTGGGACAGT-3′ R:5′-CATTCGAGGCTCCAGTGAATTC-3′ |
| IL-6 | F:5′-CCGGAGAGGAGACTTCACAG-3′ R:5'′-TCCACGATTTCCCAGAGAAC-3′ |
| HGF | F:5′-GGCTGAAAAGATTGGATCAGG-3′ R:5′-CCAGGAACAATGACACCAAGA-3′ |
| EGF | F:5′-ACAAACCAGGCTGATGATGGT-3′ R:5′-TGCAGCTGTGCAGCTATCTT-3′ |
| TGF-α | F:5′-CAGGGAGCAACACAAATGGA-3′ R:5′-AGCCTCCAGCAGACCAGAAA-3′ |
| Cyclin D1 | F:5′-TCCAAAATGCCAGAGGCGGATG-3′ R:5′-TACCATGGAGGGTGGGTTGG-3′ |
| Bax | F:5′-CGAGCTGATCAGAACCATCA-3′ R:5'-GGTCCCGAAGTAGGAGAGGA-3′ |
| Bcl-2 | F:5′-AGTACCTGAACCGGCATCTG-3′ R:5′-GCTGAGCAGGGTCTTCAGAG-3′ |
| m-Arbp | F:5′-CACTGGTCTAGGACCCGAGAAG-3′ R:5′-GGTGCCTCTGGAGATTTTCG-3′ |
| β-actin | F:5′-ACTGCGCTTCTTGCCGC-3′ R:5′-CATGACGCCCTGGTGTC-3′ |
采用Graph Pad Instat 5.0统计软件进行统计学分析。各组小鼠血清ALT水平、肝质量/初始体质量比、肝组织内细胞数量、PCNA指数和细胞因子mRNA表达水平均以x±s表示,计量资料结果符合正态分布,ALT水平、肝质量/初始体质量比、PCNA指数、每高倍视野下肝组织内细胞数量(包括肝细胞、双核细胞)以及细胞因子mRNA水平在多组间的比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠肝脏大体改变和血清ALT水平水流动力学注射后,小鼠抽搐、呼吸急促,数秒钟后恢复正常;体质量增加约10%,8 h后体质量恢复。与对照组(0 min组)比较,30 min组小鼠肝质量/初始体质量比无明显变化,8 h组明显下降(P < 0.05),1 d组和7 d组差异无统计学意义(P>0.05)。见表 2。对照组小鼠肝脏呈灰白色,表面无出血点;30 min组小鼠肝脏呈灰红色;8 h组小鼠肝脏表面出现黑红色片状出血区;1 d组小鼠肝脏表面仍可见片状出血区;3 d组小鼠出血区面积减小;7 d组小鼠肝脏呈灰白色,与对照组相似。见图 1(插页四)。30 min组和8 h组小鼠血清ALT水平明显低于对照组(P < 0.01),1 d组明显高于对照组(P < 0.01),7 d组差异无统计学意义(P>0.05)。
| (n=5, x ±s) | ||
| Group | Ratio of liver weight/original body weight |
ALT [λB/(U·L-1)] |
| Control (0 min) |
0.064±0.010 | 27.00±2.96 |
| 30 min | 0.065±0.006 | 3.40±1.82** |
| 8 h | 0.049±0.005* | 3.00±1.58** |
| 1 d | 0.064±0.005 | 250.50±62.69** |
| 3 d | 0.063±0.003 | 47.80±7.39 |
| 7 d | 0.077±0.005 | 29.20±5.26 |
| * P < 0.05,** P < 0.01 vs control group. | ||
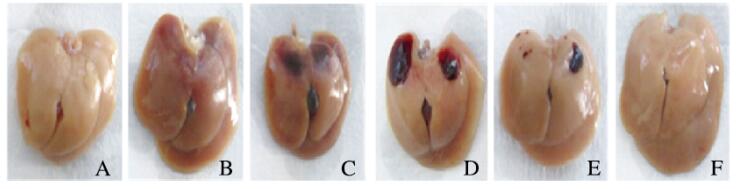
|
| A: 0 min group; B: 30 min group; C: 8 h group; D: 1 d group; E: 3 d group; F: 7 d group. 图 1 各组小鼠肝脏大体改变 Figure 1 Macroscopic changes of liversof mice in various groups |
|
|
对照组(0 min组)小鼠肝小叶结构完整,肝细胞以中央静脉为中心,沿肝细胞索呈放射状排列,肝窦清晰。30 min组小鼠出现大量肿胀的肝细胞,胞质淡染,成群分布,主要位于1区和2、3区之间;此外,肿胀区域内可见小面积出血,肝细胞索被破坏;每高倍视野下肝细胞数明显少于对照组(q=4.760,P < 0.05)。见图 2(插页五)和表 3。8 h组小鼠肿胀细胞数量较30 min组明显减少,而出血更加明显,肝细胞、肝窦内皮细胞被破坏,可见红细胞进入肿胀的肝细胞内,在出血坏死灶周围未受损区域内可见双核肝细胞及核分裂象(图 2,见插页五);与30 min组比较,肝细胞数明显增多(q=7.310,P < 0.01),双核细胞数也明显增多(q=7.200,P < 0.01)。1 d组出血坏死灶面积减小(图 2,见插页一),肝细胞数明显少于8 h组(q=4.966,P < 0.05),双核细胞数也明显少于8 h组(q=6.596,P < 0.01),但是二者与对照组比较差异无统计学意义(P>0.05)。见表 3。3 d组除靠近肝脏表面处有个别出血灶外,肝实质内出血坏死灶基本消失,肝细胞数及双核细胞数与对照组比较差异无统计学意义(P>0.05)。7 d组肝组织结构与对照组相似,肝细胞、双核细胞数量与对照组比较差异无统计学意义(P>0.05)。所有时间点均未见胆管上皮细胞的破坏。
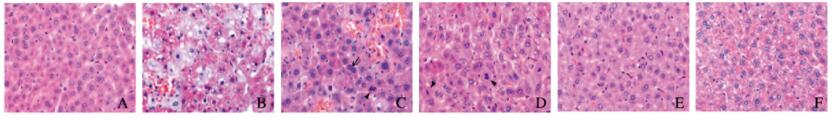
|
| A: 0 min group; B: 30 min group; C: 8 h group; D: 1 d group; E: 3 d group; F: 7 d group."→"indicated the binuclear hepatocytes; "▲"indicated the mitotic hepatocytes. 图 2 各组小鼠肝组织形态学特点(HE, ×400) Figure 2 Morphological features of liver tissue of mice in various groups (HE, ×400) |
|
|
| (n=5, x ±s) | ||
| Group | No.of hepatocytes | No.of binuclear hepatocytes |
| Control(0 min) | 78.5±7.6 | 9.4±3.2 |
| 30 min | 65.4±10.0* | 7.6±2.6 |
| 8 h | 88.0±9.7△ | 15.2±3.3△ |
| 1 d | 71.4±2.0# | 7.8±0.9## |
| 3 d | 78.0±3.7 | 6.7±0.6 |
| 7 d | 77.4±3.3 | 7.6±0.7 |
| *P < 0.05 vs control group; △P < 0.01 vs 30 min group; #P < 0.05, ##P < 0.01 vs 8 h group. | ||
免疫组织化学染色结果显示:PCNA阳性肝细胞主要位于2、3区受损区域周围。8 h组PCNA指数较对照组升高(t=4.458, P < 0.01),1 d组最高,且明显高于对照组(t=15.557, P < 0.01),7 d组与对照组比较差异无统计学意义(P>0.05)。30 min组汇管区胆管细胞PCNA指数明显高于对照组(t=3.985, P < 0.01),随后降低,7 d组与对照组比较差异无统计学意义(P>0.05)。见图 3。
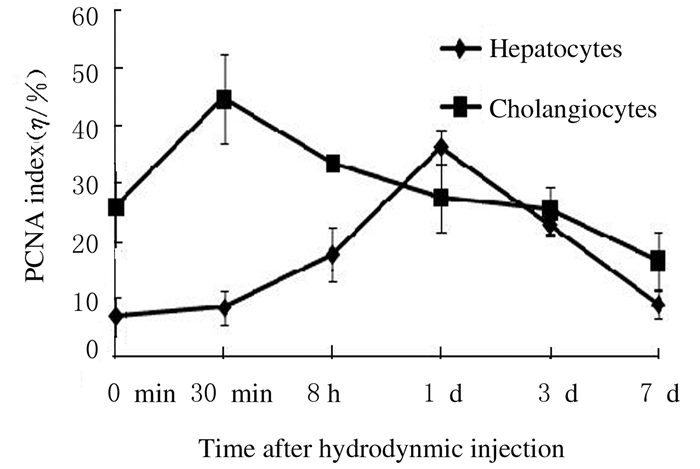
|
| 图 3 各组小鼠肝组织PCNA指数 Figure 3 PCNA indexes in liver tissue of mice in various groups |
|
|
30 min组小鼠肝组织中TNF-α mRNA水平明显高于8 h组(q=4.952,P < 0.05);8 h组仍高于7 d组(q=8.461,P < 0.01)。30 min组小鼠肝组织中IL-6 mRNA水平明显高于8 h组(q=14.750,P < 0.01),8 h组明显高于7 d组(q=6.923,P < 0.01)。30 min组各组小鼠肝组织中EGF mRNA水平明显高于8 h组(q=14.750,P < 0.01)。3 d组各组小鼠肝组织中HGF mRNA水平明显高于30 min组和8 h组(q=5.031,P < 0.05;q=4.631,P < 0.05)。8 h组和3 d组TGF-α mRNA水平均明显高于30 min组(q=4.592,P < 0.05;q=8.137,P < 0.01)。8 h和1 d组Cyclin D1 mRNA水平均明显高于7 d组(q=4.736,P < 0.05;q=5.213,P < 0.05)。30 min组各组小鼠肝组织中VEGF mRNA水平明显高于8 h组(q=13.551,P < 0.01)。1 d组Bax/Bcl-2 mRNA比值明显高于30 min组(q=5.731,P < 0.01)。见表 4。
| (n=5, x ±s) | ||||||||
| Group | TNF-α | IL-6 | EGF | HGF | TGF-α | Cyclin D1 | VEGF | Bax/Bcl-2 |
| 30 min | 3.43±1.18 | 13.45±3.09 | 1.81±0.47 | 0.39±0.08 | 0.42±0.12 | 1.28±0.25 | 2.05±0.11 | 0.50±0.17 |
| 8 h | 1.48±0.40* | 1.36±0.32** | 0.93±0.28** | 0.42±0.24 | 0.88±0.27* | 1.82±0.65 | 0.69±0.28** | 0.94±0.42 |
| 1 d | 0.68±0.14 | 0.95±0.41 | 1.00±0.08 | 0.48±0.18 | 0.82±0.28 | 2.02±0.85 | 1.00±0.23 | 1.36±0.38** |
| 3 d | 0.60±0.17 | 0.57±0.23 | 0.97±0.26 | 0.84±0.31*△ | 1.52±0.75** | 1.29±0.72# | 1.37±0.19 | 0.80±0.28 |
| 7 d | 0.40±0.15△△ | 0.46±0.07△△ | 0.88±0.44 | 0.74±0.10 | 0.79±0.17 | 0.77±0.37△# | 1.17±0.14 | 0.68±0.32 |
| *P < 0.05, * * P < 0.01 vs 30 min group; △P < 0.05, △△P < 0.01 vs 8 h group; #P < 0.01 vs 1 d group. | ||||||||
肝再生主要是由残存的健康成熟肝细胞通过增殖完成[6],除肝细胞外,胆管细胞能够通过TNF-α、IL-6和VEGF等因子的释放对组织损伤做出反应,并在上述因子的调节下,进行与胆管修复有关的增殖、凋亡以及血管生成[7]。另外,Kupffer细胞、肝星形细胞和肝窦内皮细胞可通过分泌多种细胞因子而参与肝再生,如TNF-α、IL-6以及VEGF等[8]。本实验中,注射后30 min,肝细胞数明显少于对照组,这是由于此时肝细胞肿胀、体积增大,导致单位面积内细胞数减少所致[5];注射后8 h肝细胞数以及双核细胞数明显多于30 min,肝细胞PCNA指数也明显高于30 min,这些增多的肝细胞可能是为了维持肝脏功能而产生。注射后8 h,出现弥漫的片状出血坏死灶,说明此时肝窦内皮细胞受到破坏,但是在注射后7 d,出血坏死消失,肝组织结构恢复正常。胆管细胞PCNA指数在注射后30 min高于其他时间点。以上结果说明:在水流动力学注射引起的肝损伤中,肝细胞、胆管细胞和肝窦内皮细胞均参与了肝组织修复。
本实验中,小鼠在水流动力学注射后30 min及8 h肝组织出现肝细胞肿胀、肝细胞坏死及出血,因肝细胞坏死导致8 h时肝质量/初始体质量比下降,ALT释放入血,但是因为体内注射了大量的液体,导致ALT浓度被稀释,使测得的30 min及8 h组血清ALT值明显低于对照组。注射后8 h,肝组织坏死的同时,肝细胞开始大量增殖,表现为肝细胞(含双核肝细胞)数量增加。1 d时,注射的液体已经被机体排出,1 d组小鼠血清ALT达到峰值,此时肝组织内出血、坏死灶明显减少,肝质量/初始体质量比也恢复到对照组水平,肝细胞数也与对照组相似。到注射后3 d,ALT水平、肝细胞数、PCNA指数均与对照组无差别,肝脏组织结构基本恢复正常。这与Budker等[5]的研究结果基本一致,即损伤快速出现、快速消退。
目前研究[9]认为:急性肝损伤时肝脏的再生是一个快速发生的过程,主要包括启动期、增殖期和终止期。在启动阶段,有多种细胞因子和生长因子参与调控,其中TNF-α和IL-6使肝细胞由G0期进入G1期。在四氯化碳(CCl4)诱导的肝损伤模型中,TNF-α和IL-6 mRNA表达水平在CCl4处理后1d最高;使用TNF-α抗体或者敲除TNF-α受体(TNFR1)导致TNF-α耗尽或缺失均会引起肝再生障碍,并且这与NF-κB和STAT3活化受阻从而不能诱导IL-6的表达有关[8]。肝切除后,TNF-α与非实质细胞,尤其是Kupffer细胞的受体结合,激活NF-κB信号通路产生IL-6,IL-6与肝细胞上的IL-6受体结合,激活肝细胞内STAT3以及ERK1/2信号通路,随后进行DNA复制,完成肝再生[10]。TNF-α和EGF联合可促进肝再生过程中的DNA复制。在增殖阶段,HGF、TGF-α和EGF等促有丝分裂原可通过Ras-MAPK和PI3K/AKT信号通路促进肝细胞DNA合成和细胞增殖[8]。EGF与EGFR结合后,通过促进Cyclin D1表达调节肝细胞增殖[11]。VEGF能够促进血管生成。在肝切除模型中,VEGF可以通过肝窦内皮细胞增殖,重建肝窦,促进肝细胞增殖。Bockhorn等[12]研究发现:肝切除后24 h,VEGF处理组肝细胞增殖极高,而VEGF抗体处理组肝细胞增殖几乎被完全抑制;VEGF通过与VEGFR结合刺激肝窦内皮细胞增殖,并释放IL-6和HGF,从而参与肝脏的修复。凋亡在肝脏的再生和终止阶段发挥作用[13-15]。
本实验中,注射后30 min时TNF-α、IL-6、EGF和VEGF mRNA表达水平明显升高,而Bax/Bcl-2比值较低。8 h时TNF-α和IL-6仍很高,此时Cyclin D1也升高。1 d时Cyclin D1和Bax/Bcl-2比值较高,3 d时HGF和TGF-α水平较高。本文作者推测:在水流动力学注射造成肝损伤后,快速升高的TNF-α和IL-6启动了修复的过程,使肝细胞从G0期进入G1期;EGF通过Cyclin D1使肝细胞进入增殖阶段,VEGF促进肝窦内皮细胞增殖和肝窦的修复;而后期的修复停止,可能是由促凋亡的Bax基因以及HGF和TGF-α共同发挥作用完成的。
综上所述,水流动力学注射可引起急性肝损伤,主要表现为肝细胞肿胀并形成出血、坏死灶,肝损伤后可迅速启动修复过程,损伤可在1周左右通过细胞增殖自然修复;与肝再生有关的多种细胞因子和生长因子参与修复过程,但是具体机制仍有待于进一步研究。
| [1] | Zhang GF, Budker V, Wolff JA. High levels of foreign fene expression in hepatocytes after tail vein injections of naked plasmid DNA[J]. Hum Gene Ther, 1999, 10(10): 1735–1737. DOI:10.1089/10430349950017734 |
| [2] | Huang M, Sun R, Huang Q, et al. Technical improvement and application of hydrodynamic gene delivery in study of liver diseases[J]. Front Pharmacol, 2017, 8: 591. DOI:10.3389/fphar.2017.00591 |
| [3] | Kosinska AD, Pishraft-Sabet L, WuW, et al. Low hepatitis B virus-specific T-cell response in males correlates with high regulatory T-cell numbers in murine models[J]. Hepatology, 2017, 66(1): 69–83. DOI:10.1002/hep.v66.1 |
| [4] | Zhang G, Gao X, Song YK, et al. Hydroporation as the mechanism of hydrodynamic delivery[J]. Gene Ther, 2004, 11(8): 675–682. DOI:10.1038/sj.gt.3302210 |
| [5] | Budker VG, Subbotin VM, Budker T, et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection.Ⅱ.Morphological studies[J]. J Gene Med, 2006, 8(7): 874–888. DOI:10.1002/(ISSN)1521-2254 |
| [6] | Zou Y, Bao Q, Kumar S, et al. Four waves of hepatocyte proliferation linked with three waves of hepatic fat accumulation during partial hepatectomy-induced liver regeneration[J]. PLoS One, 2012, 7(2): e30675. DOI:10.1371/journal.pone.0030675 |
| [7] | O'Hara SP, Tabibian JH, Splinter PL, et al. The dynamic biliary epithelia:molecules, pathways, and disease[J]. J Hepatol, 2013, 58(3): 575–582. DOI:10.1016/j.jhep.2012.10.011 |
| [8] | Mao SA, Glorioso JM, Nyberg SL. Liver regeneration[J]. Transl Res, 2014, 163(4): 352–362. DOI:10.1016/j.trsl.2014.01.005 |
| [9] | Tao Y, Wang M, Chen E, et al. Liver regeneration:analysis of the main relevant signaling molecules[J]. Mediat Inflamm, 2017. DOI:10.1155/2017/4256352 |
| [10] | Ogiso H, Ito H, Kanbe A, et al. Liver regeneration is impaired in mice with acute exposure to a very low carbohydrate diet[J]. Dig Dis Sci, 2017, 62: 2386–2396. DOI:10.1007/s10620-017-4651-6 |
| [11] | Collin de L' hortet A, Gilgenkrantz H, Guidotti JE. EGFR:A master piece in G1/S phase transition of liver regeneration[J]. Int J Hepatol, 2012, 2012: 476910. |
| [12] | Bockhorn M, Goralski M, Prokofiev D, et al. VEGF is important for early liver regeneration after partial hepatectomy[J]. J Surg Res, 2007, 138(2): 291–299. DOI:10.1016/j.jss.2006.07.027 |
| [13] | Takushi Y, Shiraishi M, Nozato E, et al. Expression of anti-apoptotic protein, Bcl-2, in liver regeneration after a partial hepatectomy[J]. J Surg Res, 2006, 134(1): 93–101. DOI:10.1016/j.jss.2005.11.586 |
| [14] | 高斯, 赵相轩, 卢再鸣, 等. P53调控细胞凋亡在肝细胞癌治疗中的作用[J]. 临床肝胆病杂志, 2017, 33(7): 1373–1376. |
| [15] | 周娟娟, 何文华, 甘达凯, 等. 熊果酸对TGF-β1诱导肝细胞凋亡的抑制作用及其机制[J]. 解放军医学杂志, 2017, 42(5): 383–388. DOI:10.11855/j.issn.0577-7402.2017.05.05 |
 2018, Vol. 44
2018, Vol. 44


