扩展功能
文章信息
- 李希宁, 陈晓春, 朱云飞, 曹泾楠, 邹忆婷, 杜霄, 王旗春
- LI Xining, CHEN Xiaochun, ZHU Yunfei, CAO Jingnan, ZOU Yiting, DU Xiao, WANG Qichun
- PERK信号通路对实验性绝经后骨质疏松雌性大鼠成骨细胞分化的影响
- Effect of PERK signaling pathway on osteoblast differentiation of female rats with experimental postmenopausal osteoporosis
- 吉林大学学报(医学版), 2018, 44(02): 260-264
- Journal of Jilin University (Medicine Edition), 2018, 44(02): 260-264
- 10.13481/j.1671-587x.20180210
-
文章历史
- 收稿日期: 2017-07-27
绝经后骨质疏松(postmennopausal osteoporosis, PMOP)是一种与衰老有关的常见病,主要发生在绝经后妇女[1]。PMOP核心发病机制为雌激素下降之后导致骨重建与骨吸收失衡,即成骨细胞的骨形成能力下降而破骨细胞的骨吸收能力增强,从而导致骨组织微细结构破坏、骨脆性增加以及易于骨折[2-3]。因此,PMOP的防治已成为目前迫切需要解决的公共健康问题。目前PMOP发病机制研究主要集中在成骨细胞和破骨细胞功能不平衡,导致骨质流失过多[4-6],但成骨细胞功能减弱和破骨细胞功能增强的诱导机制并未给予深入研究[7]。PERK是一种丝氨酸/苏氨酸的蛋白激酶, 内质网上错误或未折叠蛋白的聚集可引起PERK的活化, 是内质网应激的直接结果[8]。PERK基因敲除的小鼠中,成骨细胞的分化和成熟障碍并伴有Ⅰ型胶原(typeⅠcollogen,ColⅠ)分泌减少,导致骨骼发育受损,同时成骨细胞内核心结合因子1(core-binding factor a1,cbfa1)和osterix的表达下调[9-10],提示PERK很可能是一种新的调节骨骼发育和成骨细胞活动的因子。本实验通过动物实验观察PERK信号通路与成骨细胞分化和凋亡有关的转录因子、细胞因子和其他重要信息分子之间的联系,探讨PERK信号通路在PMOP发生时在成骨细胞凋亡和骨转换加速中的作用,阐明PMOP的发生机制,为制定骨质疏松的防治策略提供新的思路和理论依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器雌性Wistar大鼠(浙江大学实验动物中心),实验动物生产许可证号:SYXK(浙)2012-0178。TRIzol试剂盒(Invitrogen公司, 美国),High-Capacity cDNA ReverseTranscription Kit和Power SYBR® Green PCRMaster Mix(Applied Biosystems公司, 美国),SMARTpool reagents Nrf2和Transfection Reagent(ThermoFisher公司, 美国),Primary antibody(Santa Cruz公司, 美国),RIPA裂解液(Transgen Biotech公司, 中国)。凝胶电泳仪、电泳槽和转膜槽(Bio-Rad公司,美国),PCR仪(ThermoFisher公司,美国)。
1.2 大鼠PMOP模型复制及实验分组雌性Wistar大鼠适应饲养1周后,分为3组,每组15只:①正常对照组(大鼠不作处理);②骨质疏松组(雌性大鼠卵巢摘除);③骨质疏松治疗组(大鼠卵巢摘除后尾静脉注射雌激素)。
1.3 血液分析仪检测大鼠血清中蛋白水平大鼠饲养3个月后,采用眼球摘除法取大鼠全血样本,室温静置2h,1000r·min-1离心5 min,取上层血清样本,经血液分析仪检测各种大鼠血清中ColⅠ、碱性磷酸酶(alkaline phosphatase, ALP)和骨钙素(osteoclcin, OCN)水平。
1.4 RT-PCR法检测大鼠骨组织中PERK、ATF4、Runx2、osterix、RANKL和OPG基因表达取大鼠股骨,经液氮反复冷冻变脆敲碎,按照TRIzol试剂盒说明书提取总RNA,并用Nano-Drop分光光度计测定RNA浓度。按照High-Capacity cDNA Reverse Transcription Kit和Power SYBR® Green PCR Master Mix说明书合成cDNA和PCR扩增,GAPDH作为内参。使用StepOnePlus system software导出Ct值,基因相对表达水平采用2-(△Ct)计算。
1.5 Westernblotting法检测大鼠骨组织中PERK、ATF4、Runx2、osterix、RANKL和OPG蛋白表达取大鼠股骨,经液氮反复冷冻变脆敲碎,RIPA裂解液提取总蛋白,BCA法测定蛋白质浓度,每个泳道加入15 μg蛋白后进行SDS-PAGE电泳,电泳后转到PVDF膜,使用5%脱脂奶粉封闭PVDF膜2 h后,分别加入稀释后的抗小鼠一抗4℃孵育过夜,辣根过氧化物酶标记的二抗37℃孵育1 h,ECL显影,应用Gel Image system-1600凝胶成像系统采集图像。
1.6 HE染色观察骨组织形态学取大鼠股骨,4%多聚甲醛固定24 h,20%甲酸和15%乙二胺四乙酸脱钙14 d,各级乙醇脱水、浸蜡、包埋、切片,HE染色观察。
1.7 统计学分析采用SPSS13.0统计软件进行统计学分析。各组大鼠血清中成骨转换因子水平、骨组织中PERK、ATF4、Runx2、osterix、RANKL和OPG基因和蛋白表达水平均以x±s表示,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠血清中ColⅠ、ALP和OCN水平与对照组比较,骨质疏松组大鼠血清中ColⅠ、ALP和OCN水平明显降低(P < 0.05或P < 0.01);与骨质疏松组比较,骨质疏松治疗组大鼠血清ColⅠ、ALP和OCN水平明显升高(P < 0.01)。见表 1。
| [n=15, x±s, ρB/(μg·L-1)] | |||
| Group | Col Ⅰ | ALP | OCN |
| Control | 10.16±1.56 | 7.83±1.74 | 8.77±2.96 |
| Osteoporosis | 4.99±1.62** | 3.01±1.24* | 2.06±0.37** |
| Osteoporosis treatment | 9.02±2.13△ | 7.50±1.38△ | 8.45±1.37△ |
| *P < 0.05, * *P < 0.01 compared with control group; △P < 0.01 compared with osteoporosis group. | |||
与对照组比较,骨质疏松组大鼠骨组织中PERK和ATF4基因表达水平明显降低(P < 0.05或P < 0.01),成骨细胞转录因子Runx2和osterix基因表达水平明显降低(P < 0.05或P < 0.01),而诱导破骨细胞分化促进骨质吸收因子RANKL基因表达水平明显升高(P < 0.01);与骨质疏松组比较,骨质疏松治疗组骨组织中PERK、ATF4、Runx2和osterix基因表达水平均明显升高(P < 0.05或P < 0.01),而RANKL表达水平明显下降(P < 0.01)。见表 2。
| (n=15, x±s) | ||||||
| Group | PERK | ATF4 | Runx2 | osterix | RANKL | OPG |
| Control | 3.24±2.13 | 6.12±1.65 | 4.17±0.74 | 8.65±2.95 | 5.87±1.85 | 6.10±0.98 |
| Osteoporosis | 0.80±1.62** | 2.51±1.40* | 1.66±0.48** | 1.72±1.56** | 14.62±3.01** | 4.74±1.52 |
| Osteoporosis treatment | 3.65±2.68△△ | 6.01±0.37△△ | 3.82±1.17△ | 7.41±2.51△△ | 6.37±1.74△△ | 5.89±2.09 |
| *P < 0.05, * *P < 0.01 compared with control group; △P < 0.05, △△P < 0.01 compared with osteoporosis group. | ||||||
与对照组比较,骨质疏松组大鼠骨组织中PERK、Runx2和osterix蛋白表达水平明显降低(P < 0.05或P < 0.01),ATF4和RANKL蛋白表达水平明显升高(P < 0.05或P < 0.01);与骨质疏松组比较,骨质疏松治疗组大鼠骨组织中PERK和Runx2蛋白表达水平均明显升高(P < 0.05或P < 0.01),而RANKL蛋白表达水平明显降低(P < 0.01)。见图 1和表 3。
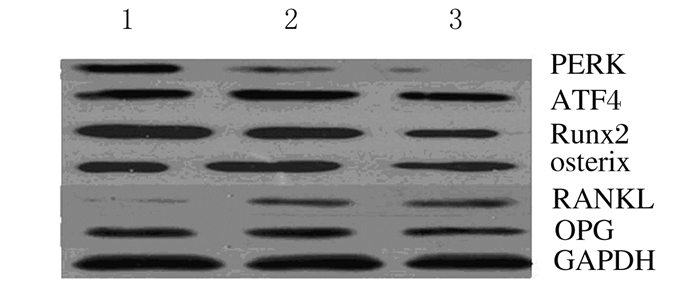
|
| Lane1: Control group; Lane 2: Osteoporosis treatment group; Lane 3: Osteoporosis group. 图 1 各组大鼠骨组织中成骨标志物蛋白表达电泳图 Figure 1 Electrophoregram of protein expressions of osteogenic markers in bone tissue of rats in various groups |
|
|
| (n=15, x±s) | ||||||
| Group | PERK | ATF4 | Runx2 | Osterix | RANKL | OPG |
| Control | 0.87±0.54 | 1.24±0.65 | 1.17±0.74 | 0.25±0.13 | 0.07±0.03 | 0.40±0.74 |
| Osteoporosis | 0.06±0.02** | 1.51±0.34* | 0.66±0.13** | 0.72±0.39** | 0.69±0.49** | 0.54±0.22 |
| Osteoporosis treatment | 0.38±0.15△△ | 1.61±0.57 | 1.12±0.68△ | 0.96±0.51△△ | 0.15±0.38△△ | 0.49±0.09 |
| *P < 0.05, * * P < 0.01 compared with control group; △P < 0.05, △△ P < 0.01 compared with osteoporosis group. | ||||||
与对照组比较,骨质疏松组大鼠股骨骨干中破骨细胞数量增多,功能活跃,骨干中出现多个骨质吸收形成的骨陷窝;与骨质疏松组比较,骨质疏松治疗组大鼠股骨骨干结构恢复正常,破骨细胞功能减弱,骨质吸收陷窝消失。见图 2(插页四)。
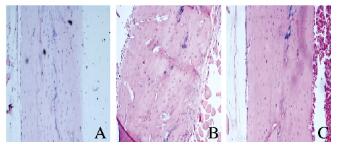
|
| A:Control group; B:Osteoporosis group; C:Osteoporosis treatment group. 图 2 各组大鼠骨组织形态学表现(HE, ×200) Figure 2 Morphology of bone tissue of rats in various groups(HE, ×200) |
|
|
PMOP的核心发病机制为雌激素下降后导致骨重建与骨吸收失衡[11]。目前对于PMOP发病机制的研究[12]主要集中在成骨细胞的活性下降和破骨细胞的活性增强,但内在的调控机制尚缺乏研究。本研究通过动物体内实验探讨PMOP发生时分子水平调控机制的异常改变,为PMOP的防治提供新的分子靶点和理论依据。
正常情况下,骨骼的结构完整和功能保持取决于破骨细胞和成骨细胞协同作用,维持骨重建与骨吸收的平衡[13]。成骨细胞作为骨形成的主要功能细胞,在分化成熟过程中会逐步表达特异性标志物,如ALP、OCN和ColⅠ等[14-15]。同时,在此过程中也会受到一系列转录因子的调控,如Runx2、Osterix/Sp7、β-catenin、ATF4、核转录因子激活蛋白1和Smads等[16-17],它们彼此协同作用,共同参与调节成骨细胞分化及骨形成。成骨细胞功能下降,导致骨转录因子和成骨因子表达减少,是导致骨质疏松形成的主要因素。PERK基因敲除的小鼠中,成骨细胞分化和成熟障碍,并伴有ColⅠ分泌减少,导致骨骼发育受损。ATF4是PERK通路下游靶分子,同时也是较早受到关注的与成骨细胞分化和骨形成相关的转录因子[18]。ATF4可协同cbfa1刺激成骨细胞内OCN的表达,提示ATF4在成骨细胞分化、成熟和骨骼发育中有重要作用[18]。研究[19]显示:体外培养功能活跃的成骨细胞中BiP和PDI表达水平明显升高,而骨质疏松症患者功能不活跃的成骨细胞中BiP和PDI表达水平明显下降,由此推测内质网应激很可能刺激成骨细胞的成骨作用。
本研究结果显示:雌性大鼠卵巢切除后,PERK和ATF4表达水平明显降低,成骨细胞转录因子Runx2和osterix表达水平明显降低,而诱导破骨细胞分化促进骨质吸收的因子RANKL表达明显升高,提示雌性大鼠卵巢切除后成骨细胞分化功能减弱而破骨细胞成熟增强引起骨质吸收增强导致出现PMOP,通过外源补充雌激素能有效缓解此种症状。本研究结果为PMOP发生机制研究提供了科学依据,为制定骨质疏松的防治策略提供了新的思路和理论依据。
| [1] | Hannafon F, Cadogan MP. Recognition and treatment of postmenopausal osteoporosis[J]. J Gerontol Nurs, 2014, 40(3): 10–14. DOI:10.3928/00989134-20140204-01 |
| [2] | Geusens P. New insights into treatment of osteoporosis in postmenopausal women[J]. RMD Open, 2015, 1(Suppl 1): e000051. DOI:10.1136/rmdopen-2015-000051 |
| [3] | Cohen A, Kamanda-Kosseh M, Recker RR, et al. Bone density after teriparatide discontinuation in premenopausal idiopathic osteoporosis[J]. J Clin Endocrinol Metab, 2015, 100(11): 4208–4214. DOI:10.1210/jc.2015-2829 |
| [4] | Shieh A, Han W, Ishii S, et al. Quantifying the balance between total bone formation and total bone resorption:An index of net bone formation[J]. J Clin Endocrinol Metab, 2016, 101(7): 2802–2809. DOI:10.1210/jc.2015-4262 |
| [5] | Hu S, Liu CC, Chen G, et al. In vivo effects of two novel ALN-EP4a conjugate drugs on bone in the ovariectomized rat model for reversing postmenopausal bone loss[J]. Osteoporos Int, 2016, 27(2): 797–808. DOI:10.1007/s00198-015-3284-x |
| [6] | McNabb BL, Vittinghoff E, Schwartz AV, et al. BMD changes and predictors of increased bone loss in postmenopausal women after a 5-year course of alendronate[J]. J Bone Miner Res, 2013, 28(6): 1319–1327. DOI:10.1002/jbmr.1864 |
| [7] | Jafari A, Qanie D, Andersen TL, et al. Legumain regulates differentiation fate of human bone marrow stromal cells and is altered in postmenopausal ssteoporosis[J]. Stem Cell Rep, 2017, 8(2): 373–386. DOI:10.1016/j.stemcr.2017.01.003 |
| [8] | Mujcic H, Nagelkerke A, Rouschop KM, et al. Hypoxic activation of the PERK/eIF2α arm of the unfolded protein response promotes metastasis through induction of LAMP3[J]. Clin Cancer Res, 2013, 19(22): 6126–6137. DOI:10.1158/1078-0432.CCR-13-0526 |
| [9] | Wei J, Sheng X, Feng D, et al. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation[J]. J Cell Physiol, 2008, 217(3): 693–707. DOI:10.1002/jcp.v217:3 |
| [10] | Yu Y, Pierciey FJ Jr, Maguire TG, et al. PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection[J]. PLoS Pathog, 2013, 9(4): e1003266. DOI:10.1371/journal.ppat.1003266 |
| [11] | Dizdar M, Irdesel JF, Dizdar OS, et al. Effects of balance-coordination, strengthening and aerobic exercises to prevent falls in postmenopausal patients with osteoporosis:A 6-month randomized parallel prospective study[J]. J Aging Phys Act, 2018, 26(1): 41–51. DOI:10.1123/japa.2016-0284 |
| [12] | Satpathy S, Patra A, Ahirwar B. Experimental techniques for screening of antiosteoporotic activity in postmenopausal osteoporosis[J]. J Complement Integr Med, 2015, 12(4): 251–266. |
| [13] | Zhao R, Xie P, Zhang K, et al. Selective effect of hydroxyapatite nanoparticles on osteoporotic and healthy bone formation correlates with intracellular calcium homeostasis regulation[J]. Acta Biomater, 2017, 59: 338–350. DOI:10.1016/j.actbio.2017.07.009 |
| [14] | Moshiri A, Oryan A, Meimandi-Parizi A. Synthesis, development, characterization and effectiveness of bovine pure platelet gel-collagen-polydioxanone bioactive graft on tendon healing[J]. J Cell Mol Med, 2015, 19(6): 1308–1332. DOI:10.1111/jcmm.2015.19.issue-6 |
| [15] | Fang Z, Yang Q, Xiong W, et al. Effect of CGRP-adenoviral vector transduction on the osteoblastic differentiation of rat adipose-derived stem cells[J]. PLoS One, 2013, 8(8): e72738. DOI:10.1371/journal.pone.0072738 |
| [16] | Choi YH, Han Y, Jin SW, et al. Pseudoshikonin I enhances osteoblast differentiation by stimulating Runx2 and Osterix[J]. J Cell Biochem, 2018, 119(1): 748–757. DOI:10.1002/jcb.26238 |
| [17] | Xu L, Kong Q. Research progress of key signaling pathways in osteoblast differentiation and bone formation regulation[J]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi, 2014, 28(12): 1484–1489. |
| [18] | Sun F, Li X, Yang C, et al. A role for PERK in the mechanism underlying fluoride-induced bone turnover[J]. Toxicology, 2014, 325: 52–66. DOI:10.1016/j.tox.2014.07.006 |
| [19] | Wei J, Sheng X, Feng D, et al. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation[J]. J Cell Physiol, 2008, 217(3): 693–707. DOI:10.1002/jcp.v217:3 |
 2018, Vol. 44
2018, Vol. 44


