扩展功能
文章信息
- 齐亚莉, 刘扬, 梁硕, 龚守良, 王志成, 刘威武
- QI Yali, LIU Yang, LIANG Shuo, GONG Shouliang, WANG Zhicheng, LIU Weiwu
- 三重靶向介导的Smac过表达对乳腺癌MDA-MB-231细胞凋亡和周期进程的影响
- Effects of Smac overexpression mediated by triple-targeting on apoptosis and cell cycle progression of breast cancer MDA-MB-231 cells
- 吉林大学学报(医学版), 2018, 44(01): 90-94
- Journal of Jilin University (Medicine Edition), 2018, 44(01): 90-94
- 10.13481/j.1671-587x.20180117
-
文章历史
- 收稿日期: 2016-12-26
2. 吉林大学公共卫生学院卫生部放射 生物学重点实验室, 吉林 长春 130021;
3. 吉林大学第二医院放射线科, 吉林 长春 130041
2. Key Laboratory of Radiobiology, Ministry of Health, School of Public Health, Jilin University, Changchun 130021, China;
3. Department of Radiology, Second Hospital, Jilin University, Changchun 130041, China
肿瘤基因-放射治疗是治疗乳腺癌的主要手段,具有广阔的应用前景[1-2]。如何增强其治疗靶向性非常重要,本文作者设想在腺病毒载体中加入人端粒酶逆转录酶(human telomerase reverse transcriptase, hTERT)启动子来增强肿瘤感染效率[3],同时将缺氧反应元件(hypoxia-response element, HREs)克隆到腺病毒载体中增强乏氧条件下的感染效率[4-5],早期生长因子(early growth response 1,Egr-1)启动子克隆到靶基因上游增强靶基因的辐射靶向效果[6-7],实现三重靶向介导凋亡诱导基因第二线粒体衍生的胱天蛋白酶激活剂(second mitochondria-derived activator of caspase,Smac),增强肿瘤细胞凋亡性死亡的比率[8]。目前国内外尚未见相关的研究报道。基于此,本研究探讨三重靶向介导的Smac过表达作用于乳腺癌MDA-MB-231细胞后对细胞周期和凋亡的影响,为临床乳腺癌放疗提供新的思路。
1 材料与方法 1.1 实验试剂、细胞系和仪器L15培养基购自美国Gibco公司,胎牛血清为杭州四季青公司产品,考马斯亮蓝蛋白定量试剂盒购自南京建成生物设计院,HRE序列、内切酶和T4连接酶购自上海生工公司,氯化钴(CoCl2)为美国Sigma公司产品,Smac和β-actin一抗及ECL发光液为美国Santa Cruz公司产品,辣根过氧化物酶标记的二抗为美国Pierce公司产品,Annexin Ⅴ-FITC试剂盒购自南京凯基生物公司。人乳腺癌MDA-MB-231细胞购自中国科学院上海细胞生物学研究所,人胚肾HEK293细胞由吉林大学公共卫生学院卫生部放射生物学重点实验室保存。X射线深部治疗机(Volcano, 荷兰Philips公司)
1.2 条件复制型腺病毒获得质粒pShuttle-Egr1-TRAIL-hTERT-E1A-E1Bp-E1B55K已经构建完成[9],pMD19T-Smac质粒由郭彩霞博士惠赠,HRE序列(5′-TGTCACGTCCTGCACGAC-3′)两端加入XhoⅠ和SmaⅤ酶切位点,将2条互补链进行合成。pMD19T-Smac质粒和pShuttle-Egr1-TRAIL-hTERT-E1A-E1Bp-E1B55K质粒分别以XhoⅠ和EcoRⅠ酶切,分别得到Smac片段和载体片段,利用T4 DNA连接酶进行连接,再将pShuttle-Egr1-Smac-hTERT-E1A-E1Bp-E1B55K质粒以XhoⅠ和SmaⅠ酶切,获得载体片段,将HRE片段连接插入后获得穿梭载体,经电转化后并同源重组形成重组腺病毒质粒,利用脂质体2000试剂将其转染到人胚肾HEK293细胞中,包装好的条件复制型腺病毒命名为CRAd.pEgr-1-Smac (CRAd.pE-Smac),以空病毒(CRAd.p)为对照。
1.3 实验分组及细胞乏氧和照射处理本实验设立对照组、CARd.pE-Smac组、乏氧组和CARd.pE-Smac +乏氧组,并根据是否进行4 Gy照射,每组又分为未照射组和照射组,共8个实验组。MDA-MB-231细胞接种于6和24孔培养板中,细胞80%~90%融合后,以感染复数(multiplicity of infection, MOI)为5的病毒感染滴度进行细胞感染,以终浓度150 μmol·L-1CoCl2模拟乏氧,乏氧24 h后采用X射线深部治疗机进行照射,剂量率为0.287 Gy·min-1。
1.4 Smac蛋白表达检测病毒感染、乏氧和照射后12 h,MDA-MB-231细胞经裂解缓冲液RIPA提取总蛋白,考马斯亮蓝试剂盒进行蛋白定量。每泳道上样50 μg总蛋白样品,进行SDS-PAGE电泳,硝酸纤维膜湿转后5%脱脂奶粉封闭1 h,β-actin和Smac一抗4℃过夜孵育,TBST洗2次,辣根过氧化物酶标记的二抗37℃孵育1 h。ECL发光液进行显影,检测表达量,并拍照进行分析。
1.5 流式细胞术检测细胞凋亡率及不同周期细胞百分率采用Annexin Ⅴ-FITC试剂盒(含有PI)双染和单染,流式细胞术检测细胞凋亡率和细胞周期百分率。按照每孔3×105个将MDA-MB-231细胞接种于24孔培养板,染毒和乏氧后进行4 Gy的X射线照射,12 h后收集细胞,PBS洗2次,按照试剂盒说明书进行染色5~15 min后,上机检测,每个样品收取1.0×104个细胞。CellQuest软件收集并分析数据,结果以细胞百分比表示。
1.6 统计学分析采用SPSS16.0统计软件进行统计学分析。各组细胞凋亡率和不同周期细胞百分率以x±s表示,组间比较采用One-Way ANOVA检验。以P < 0.05表示差异有统计学意义。
2 结果 2.1 各组MDA-MB-231细胞中Smac蛋白表达MDA-MB-231细胞经病毒感染、乏氧和4 Gy照射后6 h,CARd.pE-Smac组和CARd.pE-Smac +乏氧组细胞中Smac蛋白表达水平增加,二者之间基本一致;而CARd.pE-Smac + 4Gy组、乏氧+ 4Gy组和CARd.pE-Smac +乏氧+ 4Gy组中Smac蛋白表达水平逐渐增加,尤其以CARd.pE-Smac +乏氧+4Gy组Smac蛋白表达水平最高。见图 1。
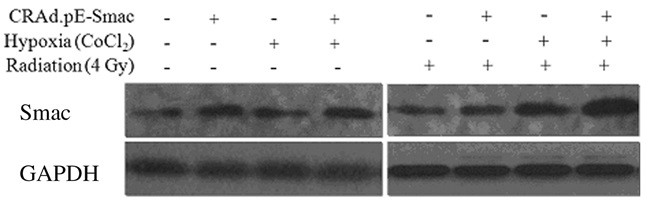
|
| 图 1 Western blotting法检测乏氧和4 Gy X射线照射后12 h MDA-MB-231细胞中Smac蛋白表达电泳图 Figure 1 Electrophoregram of expression of Smac protein in MDA-MB-231 cells 12 h after treatment of hypoxia and 4 Gy X-rays irradiation |
|
|
与对照组比较,其他各实验组细胞凋亡率均明显升高(P < 0.05或P < 0.01);与相对应的未照射组比较,4 Gy照射后各实验组细胞凋亡率均明显升高(P < 0.05或P < 0.01),CARd.pE-Smac +乏氧+4Gy组升高最为明显。见表 1和图 2。
| (n=4, x±s, η/%) | |
| Group | Apoptotic rate |
| Control | 17.29±0.40 |
| CARd.pE-Smac | 21.39±2.09* |
| Hypoxia | 21.04±1.54* |
| CARd.pE-Smac+hypoxia | 21.55±1.65* |
| Control+4 Gy irradiation | 21.01±0.76* |
| CARd.pE-Smac+4 Gy irradiation | 26.77±0.91**△ |
| Hypoxia+4 Gy irradiation | 26.37±1.47**# |
| CARd.pE-Smac +hypoxia+4 Gy irradiation | 33.36±0.62 **○ |
| * P < 0.05, ** P < 0.01 vs control group; △ P < 0.05 vs CARd.pE-Smac group; # P < 0.05 vs hypoxia group; ○ P < 0.01 vs CARd.pE-Smac+hypoxia group. | |
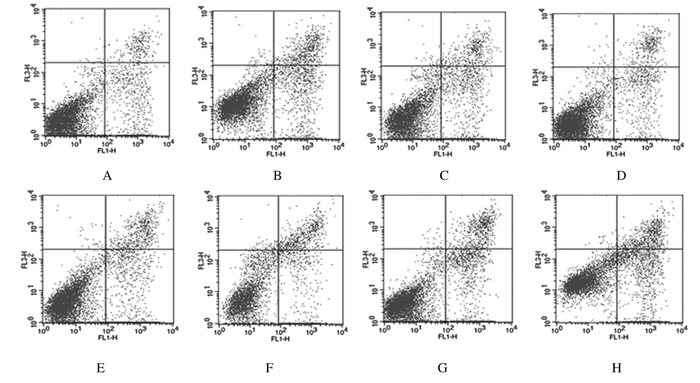
|
| A:Control group; B:CARd.pE-Smac group; C:Hypoxia group; D:CARd.pE-Smac+hypoxia group; E:Control+4 Gy irradiation group; F:CARd.pE-Smac+4 Gy irradiation group; G:Hypoxia+4 Gy irradiation group; H:CARd.pE-Smac+Hypoxia+4 Gy irradiation group. 图 2 流式细胞术检测乏氧和4 Gy照射后6 h各组MDA-MB-231细胞凋亡情况 Figure 2 Apoptosis of MDA-MB-231 cells after hypoxia and 4 Gy X-rays irradiation in various groups detected by flow cytometry |
|
|
与对照组比较,CARd.pE-Smac +乏氧组和CARd.pE-Smac+乏氧+ 4Gy组G0/G1期细胞百分率明显降低(P < 0.05),而各组S期细胞百分率均明显升高(P < 0.05),且乏氧+4Gy组和CARd.pE-Smac +乏氧+4Gy组S期细胞百分率与相对应的未照射组比较明显升高(P < 0.05);除对照+4Gy组外,其他各组G2/M期细胞百分率均较对照组明显增加(P < 0.05或P < 0.01),且CARd.pE-Smac +乏氧+4Gy组较CARd.pE-Smac +乏氧组G2/M期细胞百分率明显升高(P < 0.05)。见表 2。
| (n=4, x±s, η/%) | |||
| Group | Percentage of cells | ||
| G0/G1 | S | G2/M | |
| Control | 55.02±4.78 | 36.21±4.03 | 2.76±0.79 |
| CARd.pE-Smac | 51.16±6.14 | 42.14±1.42* | 6.71±0.34* |
| Hypoxia | 52.98±1.34 | 41.28±1.93* | 5.74±1.41* |
| CARd.pE-Smac +hypoxia | 45.47±2.80* | 42.50±1.22* | 12.03±1.29* |
| Control+4 Gyirradiation | 48.73±2.18 | 48.50±2.71* | 2.77±0.85 |
| CARd.pE-Smac+4 Gy irradiation | 49.62±1.49 | 45.85±1.55* | 6.54±1.29* |
| Hypoxia+4 Gy irradiation | 47.53±1.28 | 47.25±1.81*△ | 5.28±0.61* |
| CARd.pE-Smac+hypoxia+4 Gy irradiation | 35.54±1.19*△ | 48.76±1.21*# | 15.69±1.16 **# |
| * P < 0.05, ** P < 0.01 vs control group;△ P < 0.05 vs hypoxia group; # P < 0.05 vs CARd.pE-Smac+hypoxia group. | |||
目前,在肿瘤的基因-放射治疗研究中,靶基因的选择及其靶向性是治疗效果能否增强的关键,提高靶向性及增强目标蛋白的表达,才能有效地实现基因治疗和放射治疗的双重效应。将腺病毒载体进行基因结构的改变,使其增强对靶细胞的感染效率,最终实现靶细胞中级联的扩大效应[10-11]。hTERT启动子可介导腺病毒载体增强病毒对肿瘤的感染效率[3],在本研究中利用hTERT启动子介导条件复制型腺病毒载体。另外,相关文献[6-7, 12]证实:电离辐射能够介导Egr-1启动下游基因的表达,其根本原因是氧自由基作用于Egr-1的6个血清反应元件CarG从而发挥作用,而实体瘤内乏氧微环境导致辐射诱导自由基生成减少,影响Egr-1的效率,所以利用乏氧诱导因子反应原件HRE可介导乏氧条件下基因表达的效率[4-5]。在靶基因的选择上,本研究将Smac作为候选基因,主要是利用了Smac由线粒体释放入胞浆后,可以通过多种途径引发caspase家族的级联反应,进而促进细胞凋亡。另外,Smac与肿瘤关系密切,可在多种肿瘤组织中检测到其表达[13]。研究[8]表明:过表达Smac基因能够通过激活肿瘤细胞凋亡达到增强肿瘤细胞的放化疗敏感性,增强治疗效果。本研究中Western blotting法检测结果显示:辐射可以诱导乏氧条件下的乳腺癌MDA-MB-231细胞中Smac蛋白的高效表达,大大提高了靶向的效率。
凋亡是细胞主动死亡的重要方式之一,放疗时射线可以直接诱导凋亡,并且能够导致细胞周期检查点的阻滞,这是临床肿瘤放射治疗的基础。用一定的基因治疗策略启动细胞凋亡从而有效地抑制肿瘤细胞的生长,对肿瘤放疗有非常重要的意义[14]。Smac是一种促凋亡因子,近年来应用Smac进行抗肿瘤研究[15]表明:Smac高表达可以使肿瘤细胞凋亡,并增加肿瘤细胞放化疗的敏感性。本研究结果表明:处于乏氧状态的感染CARd.pE-Smac腺病毒的细胞,给予电离辐射则能诱导其凋亡,实现了肿瘤基因-放射治疗目的的最大化。另外,细胞凋亡可发生在细胞周期不同的时相,细胞周期的进程与凋亡有关联。本研究结果显示:病毒、乏氧和辐射以及三者联合均可明显增加S期细胞和G2/M期细胞百分率,当细胞发生G2期阻滞,其细胞的DNA损伤有充分的时间完成修复,利于细胞存活,有利于提高肿瘤细胞放射治疗效果[16]。本实验导致MDA-MB-231细胞G2/M期阻滞,可能增加了细胞放射敏感性,有利于肿瘤放疗。
综上所述,本研究实现了肿瘤靶向性,利用了肿瘤的乏氧条件和辐射诱导启动子的启动效应,从而增强了目的基因Smac过表达,对于MDA-MB-231细胞发挥凋亡诱导作用并诱发G2/M期阻滞。本研究所设计的乳腺癌基因-放射治疗综合方案,既利用了肿瘤乏氧的不利条件来解决乏氧问题,也利用hTERT启动子能够增强目的基因肿瘤靶向性的作用进一步实现肿瘤细胞的靶向性,并且利用了Egr-1启动子的辐射诱导特性,充分结合放疗时有利和不利的情况实现基因放射治疗的利益最大化。本研究为临床乳腺癌的治疗提供了新的思路。
| [1] | DeSantis C, Siegel R, Bandi P, et al. Breast cancer statistics, 2011[J]. CA Cancer J Clin, 2011, 61(6): 409–418. |
| [2] | Lara-Guerra H, Roth JA. Gene therapy for lung cancer[J]. Crit Rev Oncog, 2016, 21(1/2): 115–124. |
| [3] | Wang X, Zhou P, Sun X, et al. Modification of the hTERT promoter by heat shock elements enhances the efficiency and specificity of cancer targeted gene therapy[J]. Int J Hyperthermia, 2016, 32(3): 244–253. DOI:10.3109/02656736.2015.1128569 |
| [4] | Okino ST, Chichester CH, Whitlock JP Jr. Hypoxia-inducible mammalian gene expression analyzed in vivo at a TATA-driven promoter and at an initiator-driven promoter[J]. J Biol Chem, 2004, 273(37): 23837–23843. |
| [5] | Kwon OJ, Kim PH, Huyn S, et al. A hypoxia-and {alpha}-fetoprotein-dependent oncolytic adenovirus exhibits specific killing of hepatocellular carcinomas[J]. Clin Cancer Res, 2010, 16(24): 6071–6082. DOI:10.1158/1078-0432.CCR-10-0664 |
| [6] | Liu LL, Smith MJ, Sun BS, et al. Combined IFN-gamma-endostatin gene therapy and radiotherapy attenuates primary breast tumor growth and lung metastases via enhanced CTL and NK cell activation and attenuated tumor angiogenesis in a murine model[J]. Ann Surg Oncol, 2009, 16(5): 1403–1411. DOI:10.1245/s10434-009-0343-6 |
| [7] | Li Y, Guo C, Wang Z, et al. Enhanced effectes of TRAIL-endostatin-based double-gene-radiotherapy on suppressing growth, promoting apoptosis and inducing cell cycle arrest in vascular endothelial cells[J]. J Huazhong Univ Sci Technolog Med Sci, 2012, 32(2): 167–172. DOI:10.1007/s11596-012-0030-x |
| [8] | Pluta P, Cebula-Obrzut B, Ehemann V, et al. Correlation of Smac/DIABLO protein expression with the clinico-pathological features of breast cancer patients[J]. Neoplasma, 2011, 58(5): 430–435. DOI:10.4149/neo_2011_05_430 |
| [9] | 王宏芳, 吴嘉慧, 刘纯岩, 等. 携带TRAIL基因的条件复制型腺病毒载体的构建及其辐射诱导表达[J]. 吉林大学学报:医学版, 2014, 40(4): 699–704. |
| [10] | Yang SW, Chanda D, Cody JJ, et al. Conditionally replicating adenovirus expressing TIMP2 increases survival in a mouse model of disseminated ovarian cancer[J]. PLoS One, 2011, 6(10): e25131. DOI:10.1371/journal.pone.0025131 |
| [11] | Zheng FQ, Xu Y, Yang RJ, et al. Combination effect of oncolytic adenovirus therapy and herpes simplex virus thymidine kinase/ganciclovir in hepatic carcinoma animal models[J]. Acta Pharmacol Sin, 2009, 30(5): 617–627. DOI:10.1038/aps.2009.33 |
| [12] | Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene:a phase Ⅰ study in patients with solid tumors[J]. J Clin Oncol, 2004, 22(4): 592–601. DOI:10.1200/JCO.2004.01.227 |
| [13] | Anguiano-Hernandez YM, Chartier A, Huerta S. Smac/DIABLO and colon cancer[J]. Anticancer Agents Med Chem, 2007, 7(4): 467–473. DOI:10.2174/187152007781058631 |
| [14] | Ledgerwood EC, Morison IM. Targeting the apoptosome for cancer therapy[J]. Clin Cancer Res, 2009, 15(2): 420–424. DOI:10.1158/1078-0432.CCR-08-1172 |
| [15] | Fandy TE, Shankar S, Srivastava RK. Smac/DIABLO enhances the therapeutic potential of chemotherapeutic drugs and irradiation, and sensitizes TRAIL-resistant breast cancer cells[J]. Mol Cancer, 2008, 7: 60. DOI:10.1186/1476-4598-7-60 |
| [16] | Deplanque G, Céraline J, Mah-Becherel MC, et al. Caffeine and the G2/M block override:a concept resulting from a misleading cell kinetic delay, independent of functional p53[J]. Int J Cancer, 2001, 94(3): 363–369. DOI:10.1002/(ISSN)1097-0215 |
 2018, Vol. 44
2018, Vol. 44


