扩展功能
文章信息
- 于雷, 王志成, 王铁君
- YU Lei, WANG Zhicheng, WANG Tiejun
- 咖啡因联合电离辐射对沉默Chk-1肝癌干细胞的增殖抑制和凋亡诱导作用
- Inhibitory effect of caffeine combined with ionizing radiation on proliferation of hepatocellular carcinoma stem cells silenced by Chk-1 and its apoptosis-induced effect
- 吉林大学学报(医学版), 2018, 44(01): 52-57
- Journal of Jilin University (Medicine Edition), 2018, 44(01): 52-57
- 10.13481/j.1671-587x.20180110
-
文章历史
- 收稿日期: 2017-06-02
2. 吉林大学公共卫生学院 卫生部放射生物学重点实验室, 吉林 长春 130021
2. Key Laboratory of Radiobiology, Ministry of Health, School of Public Health, Jilin University, Changchun 130021, China
肝癌是全球5大常见肿瘤之一,放射治疗已经成为原发性肝癌的治疗手段,并在我国的原发性肝癌诊治指南得到推荐[1]。但是,由于肿瘤干细胞(cancer stem cell,CSC)的存在,导致肿瘤的复发和转移,而且是肿瘤放疗抵抗的重要原因之一[2],因此如何提高肝癌放疗效果成为目前研究的热点之一。肿瘤细胞受到辐射后,DNA的损伤如未修复则发生细胞凋亡[3],检查点激酶1(checkpoint kinase1,Chk-1)是一种进化上高度保守的丝氨酸/苏氨酸蛋白激酶,可有效调节细胞周期进程,具有重要的生理功能,当Chk-1被抑制后,则调控周期阻滞的功能消失,使其成为肿瘤治疗的重要靶点。另外,咖啡因(caffeine)是一种茶叶、可可豆和咖啡果中的提取物,近期研究显示其具有抗肿瘤作用,并且取得了较好的效果[4-6]。本研究探讨咖啡因增强辐射对沉默了Chk-1的CD133+肝癌HepG2细胞增殖、周期和凋亡的作用,为改善肝癌细胞放疗抵抗提供新的思路。
1 材料与方法 1.1 细胞株及主要试剂人肝癌HepG2细胞和人肾上皮293T细胞由吉林大学公共卫生学院王志成博士惠赠。培养血清及高糖DMEM培养基(江苏恩莫阿塞生物技术有限公司),DMEM/F12培养基、靶向沉默Chk-1的慢病毒载体pGIPZ-Chk-1 shRNA(第6外显子)、非靶对照载体pGIPZ-control shRNA、质粒pMD2G和pSPAX2由吉林大学卫生部放射生物学重点实验室保存;转染试剂Lipofectamine 2000和B27(美国Invitrogen公司),puromycin、MTT试剂、咖啡因和碘化丙啶(PI)(美国Sigma公司),重组人碱性成纤维细胞生长因子(bFGF)和重组人表皮生长因子(EGF)(美国Peprtech公司),牛血清白蛋白(BSA)(美国Gbico公司),AnnexinⅤ-FITC凋亡检测试剂盒(南京凯基生物公司),CD133一抗(美国Abcam公司),GAPDH、Chk-1兔多克隆抗体和ECL发光试剂盒(美国Santa Cruz公司),辣根过氧化物酶标记的抗兔二抗(北京中山金桥公司),其他试剂为国产分析纯。
1.2 沉默Chk-1的HepG2细胞模型的建立293T细胞接种6孔板,待80%~90%融合时,利用Lipofectamine 2000转染试剂将pGIPZ-Chk-1 shRNA、pMD2G和pSPAX2质粒共转染到293T细胞中,48和72 h收取上清液并利用0.45 μm滤膜过滤后加到培养于6孔板80%~90%融合的HepG2细胞中,共感染2次。通过观察绿色荧光的状态判定感染的效率,并加入50 g·L-1的puromycin 10 μL进行阳性筛选,将获得的细胞命名为HepG2-Chk-1,以pGIPZ-control作为非靶对照,命名为HepG2-control,冻存备用。
1.3 Western blotting法检测Chk-1蛋白的表达收集HepG2、HepG2-Chk-1和HepG2-control组细胞,利用RIPA裂解细胞,总蛋白提取后进行定量,按照25 μg蛋白上样进行SDS-PAGE电泳,将蛋白转到硝酸纤维膜后,利用TBST配置的5%脱脂奶粉封闭1 h,4℃过夜GAPDH和Chk-1一抗孵育。含有0.05%Tween 20的TBST洗3次,每次5 min,辣根过氧化物酶标记的二抗37℃下孵育1 h,TBST洗3次,利用化学发光试剂ECL进行显像,并进行拍照分析。
1.4 沉默Chk-1的肝癌干细胞的获得参照文献[7-8]进行肝癌干细胞的悬浮培养,DMEM/F12培养基中加入20 μg·L-1 EGF、10 μg·L-1 bFGF、2% B27、0.4% BSA、100 U· mL-1青霉素和100 mg·L-1链霉素,于37℃、5% CO2条件下培养,约6 d可形成悬浮球,采用Western blotting法检测CD133蛋白表达确定是否为肝癌干细胞,命名为S-HepG2-Chk-1细胞(沉默Chk-1的肝癌干细胞)和S-HepG2-control细胞(肝癌干细胞)。
1.5 细胞分组和照射S-HepG2-Chk-1和S-HepG2-control细胞分别分为对照组、咖啡因(0.2 mmol·L-1)组、4 Gy组和4 Gy+咖啡因组。X射线照射采用X-RAD320生物辐照仪(美国PXI公司),照射条件:电压180 kV,电流12.0 mA,靶皮距70 cm,剂量率1.0 Gy·min-1,总剂量为4 Gy。
1.6 MTT法检测细胞增殖活性收集各组肝癌干细胞悬浮球,0.25%胰蛋白酶消化后按照1× 104个/孔接种96孔板,参照1.4中的方法继续培养,12 h后加入终浓度为0.2 mmol·L-1的咖啡因,24 h后进行X射线照射,计为0 h,分别于4、12、24和48 h加入MTT试剂,再培养4 h后吸去上清,加DMSO到孔底结晶处,于酶标仪上490 nm处测定吸光度[A(490)]值,代表其增殖活性。
1.7 流式细胞术检测细胞周期收集肝癌干细胞悬浮球,0.25%胰蛋白酶消化后按照4 × 105个/孔接种24孔板,参照1.4中的方法继续培养12 h后加入终浓度为0.2mmol·L-1的咖啡因,24 h后进行X射线照射,24 h后收集细胞,1 mol·L-1PBS洗2次,每个管中加入200 μLPI和100 μL RNaseA,轻微震荡混匀,室温条件避光20 min,上机收细胞,CellQuest软件收集,ModFit软件分析数据,结果以各期细胞百分率表示。
1.8 流式细胞术检测细胞凋亡率收集各组肝癌干细胞悬浮球,0.25%胰蛋白酶消化后按照4 × 105个/孔接种24孔板,参照1.4中的方法继续培养,12 h后加入终浓度为0.2 mmol·L-1的咖啡因[9],24 h后进行X射线照射,24 h后收细胞,1 mol·L-1的PBS洗2次,每个管中加入5 μL PI和5 μL Annexin V-FITC试剂,混匀后避光10 min,上机收细胞,CellQuest软件收集数据,ModFit软件分析,细胞凋亡率以百分率表示。
1.9 统计学分析采用SPSS 13.0统计软件进行统计学分析。细胞增殖活性、细胞周期百分率和凋亡率均符合正态分布,以x±s表示,两两比较采用两独立样本t检验。以P < 0.05表示差异有统计学意义。
2 结果 2.1 沉默Chk-1的HepG2细胞模型的鉴定利用转染293T细胞获得的慢病毒感染HepG2细胞后获得稳定沉默Chk-1的细胞模型,通过Western blotting法检测Chk-1蛋白的表达。HepG2和非靶对照shRNA的HepG2细胞中均可见Chk-1蛋白的表达,而靶向沉默Chk-1的HepG2细胞中Chk-1蛋白低表达,表明本研究获得的沉默Chk-1的HepG2细胞模型成功。见图 1。
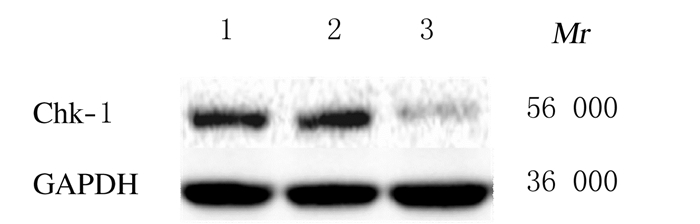
|
| Lane 1:HepG2cells; Lane 2:HepG2-control shRNA cells; Lane 3:HepG2-Chk-1 shRNA cells. 图 1 Western blotting法检测Chk-1蛋白表达电泳图 Figure 1 Electrophoregram of Chk-1 protein expression measured by Western blotting method |
|
|
根据Yoshikawa等[10]报道,肝癌干细胞表面标志为CD133。在悬浮培养6 d后,收集细胞采用Western blotting法检测,HepG2-control和HepG2-Chk-1细胞中可见CD133蛋白表达,而将2种细胞悬浮培养后,CD133蛋白均高表达,提示细胞中高比例的CD133+细胞为肝癌干细胞。见图 2。

|
| Lane 1:HepG2-controlcells; Lane 2:HepG2-Chk-1 cells; Lane 3:S-HepG2-control cells; Lane 4:S-HepG2-Chk-1 cells. 图 2 Western blotting法检测CD133蛋白表达电泳图 Figure 2 Electrophoregram of CD133 protein expressions measured by Western blotting method |
|
|
采用MTT比色法检测细胞增殖活性。在相同时间点,与对照组比较,同一种细胞的增殖活性均明显降低(除了4 h时S-HepG2-control细胞)(P < 0.05或P < 0.01),且以咖啡因+ 4 Gy组的细胞活性最低;与S-HepG2-control细胞比较,48 h时对照组,4、12、24和48 h时咖啡因组,12、24和48 h时4 Gy组,以及4、12、24和48 h时咖啡因+4 Gy组S-HepG2-Chk-1细胞活性均明显降低(P < 0.01)。见表 1。
| (n=6, x±s) | |||||
| Group | Cells | A (490) value | |||
| (t/h) 4 | 12 | 24 | 48 | ||
| Control | S-HepG2-control | 0.62±0.02 | 0.68±0.01 | 0.74±0.02 | 0.85±0.01 |
| S-HepG2-Chk-1 | 0.62±0.01 | 0.67±0.02 | 0.71±0.01 | 0.80±0.01△△ | |
| Caffeine | S-HepG2-control | 0.60±0.01 | 0.64±0.01* | 0.68±0.01* | 0.69±0.02** |
| S-HepG2-Chk-1 | 0.56±0.02*△ | 0.61±0.01*△ | 0.63±0.02**△ | 0.55±0.02**△△ | |
| 4 Gy | S-HepG2-control | 0.54±0.02** | 0.58±0.01** | 0.63±0.01** | 0.70±0.01** |
| S-HepG2-Chk-1 | 0.50±0.01** | 0.53±0.01**△ | 0.57±0.01**△ | 0.59±0.01**△△ | |
| Caffeine + 4 Gy | S-HepG2-control | 0.41±0.02** | 0.42±0.01** | 0.54±0.01** | 0.56±0.01** |
| S-HepG2-Chk-1 | 0.11±0.01**△△ | 0.12±0.01**△△ | 0.21±0.02**△△ | 0.31±0.01**△△ | |
| * P < 0.05, * * P < 0.01 vs control group; △ P < 0.05, △△ P < 0.01 vs S-HepG2-control cells. | |||||
与对照组比较,咖啡因组S期和G2/M期细胞百分率明显增加(P < 0.05或P < 0.01),4 Gy组G0/G1期和G2/M期细胞百分率明显增加(P < 0.01);咖啡因+ 4 Gy组S期细胞百分率明显降低,而G2/M期细胞百分率明显增加(P < 0.05或P < 0.01)。与S-HepG2-control细胞比较,S-HepG2-Chk-1细胞G2/M期细胞百分率均明显降低(P < 0.05或P < 0.01)。见表 2。
| (n=3, x±s, η/%) | ||||
| Group | Cells | Percentage of cells | ||
| G0/G1 | S | G2/M | ||
| Control | S-HepG2-control | 47.07±0.93 | 49.57±0.92 | 3.36±0.21 |
| S-HepG2-Chk-1 | 53.95±0.35△ | 45.00±0.34△ | 1.05±0.06? | |
| Caffeine | S-HepG2-control | 32.80±1.49** | 60.43±1.62* | 6.77±0.31** |
| S-HepG2-Chk-1 | 45.31±0.63**△ | 49.24±0.71*△ | 5.45±0.40*△ | |
| 4 Gy | S-HepG2-control | 50.20±0.66* | 31.84±1.33** | 17.96±0.69** |
| S-HepG2-Chk-1 | 57.03±0.28**△ | 33.00±0.47** | 9.97±0.71**△△ | |
| Caffeine+4 Gy | S-HepG2-control | 34.67±0.92** | 39.03±0.55** | 26.30±1.11** |
| S-HepG2-Chk-1 | 50.14±0.87*△△ | 30.85±0.49**△△ | 19.01±0.47**△ | |
| * P < 0.05, * * P < 0.01 vs control group; △ P < 0.05, △△ P < 0.01 vs S-HepG2-control cells. | ||||
与对照组比较,咖啡因组、4 Gy组和咖啡因+ 4 Gy组细胞凋亡率均明显增加(P < 0.05或P < 0.01),且咖啡因+ 4 Gy组增加最明显;与S-HepG2-control细胞比较,各组S-HepG2-Chk-1细胞凋亡率均明显增加(P < 0.05或P < 0.01)。见表 3和图 3。
| (n=3, x±s, η/%) | ||
| Group | Apoptotic rate | |
| S-HepG2-control | S-HepG2-Chk-1 | |
| Control | 13.47±0.56 | 21.70±0.63△△ |
| Caffeine | 17.45±0.50* | 25.33±1.42*△ |
| 4 Gy | 24.95±0.82** | 29.47±1.68*△ |
| Caffeine+4 Gy | 30.33±0.71** | 49.40±0.71**△△ |
| * P < 0.05, * * P < 0.01 vs control group; △ P < 0.05, △△ P < 0.01 vs S-HepG2-control cells. | ||
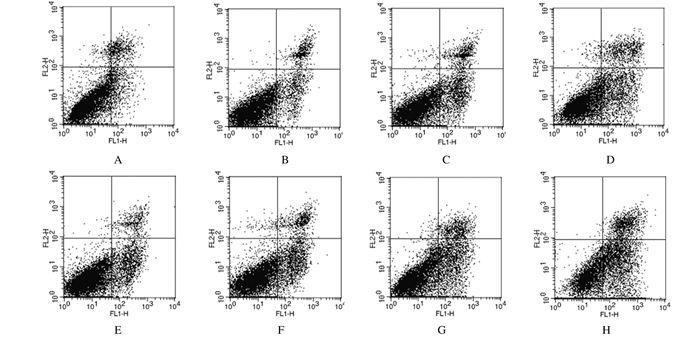
|
| A-D:S-HepG2-control cells; E-H:S-HepG2-Chk-1 cells; A, E:Control group; B, F:Caffeine group; C, G:4 Gy group; D, H:Caffeine+4 Gy group. 图 3 流式细胞术检测各组肝癌干细胞凋亡率 Figure 3 Apoptotic rates of hepatocellular carcinoma stem cell in various groups detected by flow cytometry |
|
|
CSC为肿瘤组织中存在的非常小比例的细胞,具有无限自我更新和增殖能力,维持细胞群的生命力。CSC长时间处于休眠状态,对肿瘤的存活、增殖、转移和复发具有重要作用,同时也是肿瘤放疗抵抗和化疗多药耐药的影响因素之一,以CSC为靶点的肿瘤治疗策略越来越引起重视[11-12]。原发性肝癌包括肝细胞癌和肝内胆管癌,有学者认为可能部分起源于肝癌干细胞。目前,识别CSC的表面标志物被认为是鉴定CSC的重要途径。肝癌干细胞具有多个表面标志物,如CD133、CD90、CD44、OV6和EpCAM等[13]。在本研究中悬浮培养6 d后,检测肝癌干细胞表面标志物CD133蛋白表达结果显示:与未悬浮的相同细胞比较,CD133蛋白呈高表达,提示有高比例的肝癌干细胞存在,为后续实验提供了保障。
肿瘤放疗时损伤细胞的一个重要的机制就是诱导DNA损伤,继而导致细胞最终走向死亡。DNA损伤时细胞周期Chk-1/2在限制细胞周期进展的DNA损伤反应通路中发挥重要作用[14]。肿瘤临床放疗时,往往因肿瘤细胞逃避了凋亡,使得以诱导细胞凋亡为目的的治疗方案效果降低,这与细胞周期检查点的活化关系密切。当细胞遭受DNA损伤剂作用时,Chk1被激活,致使细胞停留在S和G2/M期检查点,保证DNA有充足的时间完成修复,从而维持基因组的稳定性[15]。本研究中靶向Chk-1第6外显子的靶向RNAi慢病毒pGIPZ-Chk-1感染肝癌细胞HepG2后,Western blotting法检测结果显示:Chk-1蛋白表达大大降低,具有较好的沉默效果,而对照序列的慢病毒则不影响Chk-1蛋白的表达。另外,流式细胞术检测Chk-1被沉默的HepG2细胞和对照序列的HepG2细胞周期结果显示:G2/M期细胞百分率明显降低,表明经过悬浮培养后沉默Chk-1的细胞更少的处于G2/M期。
2013年,美国国家综合癌症网络(NCCN)肝癌诊治指南推荐:原发性肝癌患者无论肿瘤位于何处,都适合外照射放疗。我国也在肝癌诊治指南中推荐肝癌放疗,而肿瘤放疗与其他疗法的联合应用则具有更高的治疗效果。咖啡因广泛存在于茶叶、咖啡和其他饮料中,有研究[16]表明:咖啡因能够通过诱发细胞凋亡和抑制增殖实现抗肿瘤效应。放疗也可以诱导肿瘤细胞凋亡,因此本研究将二者结合以增加彼此的诱导凋亡作用,并且以沉默了Chk-1的肝癌干细胞为靶细胞。沉默了Chk-1后G2/M期细胞比例减少,而该细胞周期是对X射线最敏感的期,这将对放射效果产生影响,但从本研究结果来看,二者协同作用于沉默Chk-1的肝癌干细胞能明显提高其增殖抑制和凋亡促进效果。另外,咖啡因对于细胞周期的影响主要集中在S相延迟和G2/M期阻滞,而4 Gy照射则诱导G0/G1和G2/M期阻滞,对于逆转因缺少Chk-1调控周期检查点监控失调具有一定的作用。
综上所述,由沉默了Chk-1的HepG2细胞悬浮培养获得的肝癌干细胞G2/M期比例降低,本身不利于细胞维持正常化,但对于肿瘤来说,则有利于以此为突破口进行靶向治疗。咖啡因和电离辐射均可诱导细胞凋亡,二者联合应用大大增强了单一治疗的效果。本研究结果为肝癌的放射治疗提供了新的实验数据和思路。
| [1] | 曾昭冲. 原发性肝癌放射治疗现状及前景[J]. 中国实用外科杂志, 2014, 34(8): 699–702. |
| [2] | Burgess DJ. Stem cells:competitive behaviour of cancer mutations[J]. Nat Rev Cancer, 2014, 14(1): 5. DOI:10.1038/nrc3652 |
| [3] | Zhou ZR, Yang ZZ, Wang SJ, et al. The Chk1 inhibitor MK-8776 increases the radiosensitivity of human triple-negative breast cancer by inhibiting autophagy[J]. Acta Pharmacol Sin, 2017, 38(4): 513–523. DOI:10.1038/aps.2016.136 |
| [4] | Fujimaki S, Matsuda Y, Wakai T, et al. Blockade of ataxia telangiectasia mutated sensitizes hepatoma cell lines to sorafenib by interfering with Akt signaling[J]. Cancer Lett, 2012, 319(1): 98–108. DOI:10.1016/j.canlet.2011.12.043 |
| [5] | Kawano Y, Nagata M, Kohno T, et al. Caffeine increases the antitumor effect of Cisplatin in human hepatocellular carcinoma cells[J]. Biol Pharm Bull, 2012, 35(3): 400–407. DOI:10.1248/bpb.35.400 |
| [6] | Al-Ansari MM, Aboussekhra A. Caffeinemediates sustained inactivation of breast cancer-associated myofibroblasts via up-regulation of tumor suppressor genes[J]. PLoS One, 2014, 9(3): e90907. DOI:10.1371/journal.pone.0090907 |
| [7] | Wang L, Huang X, Zheng X, et al. Enrichment of prostate cancer stem-like cells from human prostate cancer cell lines by culture inserum-free medium and chemoradiotherapy[J]. Int J Biol Sci, 2013, 9(5): 472–479. DOI:10.7150/ijbs.5855 |
| [8] | Peng YC, Lu SD, Zhong JH, et al. Combination of 5-fluorouracil and 2-morphilino-8-phenyl-4H-chromen-4-one may inhibit livercancer stem cell activity[J]. Tumour Biol, 2016, 37(8): 10943–10958. DOI:10.1007/s13277-016-4915-3 |
| [9] | 刘寒旸, 宋军, 周艳, 等. 咖啡因与低剂量阿司匹林协同抑制结肠癌细胞生长的机制[J]. 中国癌症防治杂志, 2016, 8(6): 337–343. |
| [10] | Yoshikawa S, Zen Y, Fujii T, et al. Characterization of CD133+ parenchymal cells in the liver:histology and culture[J]. World J Gastroenterol, 2009, 15(39): 4896–4906. DOI:10.3748/wjg.15.4896 |
| [11] | Kumazawa S, Kajiyama H, Umezu T, et al. Possible association between stem-like hallmark and radioresistance in human cervical carcinoma cells[J]. J Obstet Gynaecol Res, 2014, 40(5): 1389–1398. DOI:10.1111/jog.12357 |
| [12] | Han X, Du F, Jiang L, et al. A2780 human ovarian cancer cells with acquired paclitaxel resistance display cancer stem cell properties[J]. Oncol Lett, 2013, 6(5): 1295–1298. DOI:10.3892/ol.2013.1568 |
| [13] | 章健, 来维洁, 周秀梅, 等. 靶向肝癌干细胞的肿瘤治疗进展[J]. 中国细胞生物学学报, 2017, 39(1): 87–96. DOI:10.11844/cjcb.2017.01.0260 |
| [14] | Garrett MD, Collins I. Anticancer therapy with checkpoint inhibitors:what, where and when?[J]. Trends Pharmacol Sci, 2011, 32(5): 308–316. DOI:10.1016/j.tips.2011.02.014 |
| [15] | Carrassa L, Chilà R, Lupi M, et al. Combined inhibition of Chk1 and Wee1:in vitro synergistic effect translates to tumor growth inhibition in vivo[J]. Cell Cycle, 2012, 11(13): 2507–2517. DOI:10.4161/cc.20899 |
| [16] | Ku BM, Lee YK, Jeong JY, et al. Caffeine inhibits cell proliferation and regulates PKA/GSK3β pathways in U87MG human glioma cells[J]. Mol Cells, 2011, 31(3): 275–279. DOI:10.1007/s10059-011-0027-5 |
 2018, Vol. 44
2018, Vol. 44


