扩展功能
文章信息
- 冯安琪, 刘楠, 朴美花, 苑野, 裴爱月, 刘娅, 冯春生
- FENG Anqi, LIU Nan, PIAO Meihua, YUAN Ye, PEI Aiyue, LIU Ya, FENG Chunsheng
- 异氟醚对缺锌APP/PS1转基因阿尔茨海默病模型小鼠海马神经元凋亡的影响
- Effect of isoflurane anesthesia on hippocampus neuroapoptosis in zinc-deficient APP/PS1 transgenic mice of Alzheimer's disease models
- 吉林大学学报(医学版), 2018, 44(01): 24-29
- Journal of Jilin University (Medicine Edition), 2018, 44(01): 24-29
- 10.13481/j.1671-587x.20180105
-
文章历史
- 收稿日期: 2017-06-13
2. 吉林大学第一医院内镜中心, 吉林 长春 130021;
3. 吉林大学公共卫生学院营养与食品卫生学教研室, 吉林 长春 130021
2. Department of Endoscope Center, First Hospital, Jilin University, Changchun 130021, China;
3. Department of Nutrition and Food Hygiene, school of Public Health, Jilin University, Changchun 130021, China
每年全球近千万阿尔茨海默病(Alzheimer’ s disease,AD)患者接受全身麻醉。研究[1-3]显示:常用吸入麻醉药异氟醚能够增加海马β淀粉样蛋白(amyloid β-peptide,Aβ)聚集和Tau蛋白过度磷酸化,加重神经元凋亡等AD神经病理进程,但其相关机制及影响因素尚不清楚。锌是中枢神经系统重要微量金属元素之一,在参与神经递质的合成和维持神经细胞的结构及生理功能等方面发挥重要作用[4-7]。研究[8-15]显示:缺锌能够引起细胞凋亡,导致包括AD在内的多种神经系统变性疾病的发生。流行病学调查[8, 15]显示:AD患者缺锌发生率高,约25% AD人群存在血锌水平降低。然而,缺锌是否为异氟醚促进AD进展的风险因素,临床相关异氟醚麻醉对缺锌AD患者的神经病理影响尚不完全清楚。为此,本研究拟采用缺锌APP/PS1转基因小鼠模型,探讨异氟醚麻醉对缺锌AD小鼠海马神经元凋亡和Aβ表达的影响及其相关机制。
1 材料与方法 1.1 实验动物、试剂和抗体APP/PS1转基因小鼠购自中国医学科学院医学实验动物研究所北京协和医学院比较医学中心(动物合格证号:11400700087484),在标准实验室条件下(白天/黑夜12 h:12 h,温度22℃, 湿度60%,4~5只/笼)饲养,出生2周后行基因型检测。饲料是以AIN 93标准饲料为基础的半纯化饲料,购自天津军事医学科学院卫生学环境医学研究所。应用电感耦合等离子体质谱(ICP-MS)检测饲料锌含量。
鼠单克隆抗Aβ抗体、鼠单克隆抗Aβ40抗体和兔单克隆抗Bax抗体(Abcam公司, 美国),鼠单克隆抗β肌动蛋白抗体、鼠单克隆抗GAPDH抗体和DAPI(Sigma公司, 美国),鼠单克隆抗Aβ42抗体(COVANCE公司, 美国),Caspase-3抗体(Cell Signaling Technology公司, 美国),鼠抗NeuN单克隆抗体(Millipore公司, 美国),异氟醚(Abbott公司,美国),蛋白酶抑制剂(Roche公司,瑞士)。
1.2 实验动物分组8和9月龄APP/PS1转基因小鼠随机分为4组,每组24只。①常锌组(ZA组):8月龄小鼠给予常锌饲料(锌含量30 mg·kg-1)2个月;②常锌+异氟醚组(ZA+Iso组):8月龄小鼠给予常锌饲料2个月后接受1.4%异氟醚麻醉2 h;③缺锌组(ZD组):9月龄小鼠给予低锌饲料(锌含量1 mg·kg-1)1个月;④缺锌+异氟醚组(ZD+Iso组):9月龄小鼠给予低锌饲料1个月后接受1.4%异氟醚麻醉2 h。
1.3 小鼠血清锌和脑锌水平测定应用ICP-MS分别检测8月龄(建模前)和10月龄(建模后)小鼠血清锌水平,成功建立缺锌AD模型后检测各组10月龄小鼠脑锌水平。
1.4 异氟醚麻醉常锌+异氟醚组和缺锌+异氟醚组小鼠在特制麻醉箱中接受全麻,吸入3%异氟醚和3 L·min-1氧气的混合气体,当小鼠翻正反射消失后持续吸入1.4%异氟醚和1 L·min-1氧气麻醉2 h。麻醉箱中放置体积分数为0.03%的CO2、加温毯和红外线灯。麻醉过程中小鼠保持自主呼吸,监测血压、心率、血氧饱和度和肛温。应用红外线吸收装置持续监测麻醉箱流出气体中异氟醚、氧气和CO2的浓度。常锌组和缺锌组小鼠在相同麻醉箱中持续吸入相同浓度和流量的氧气2 h。麻醉结束后吸入3 L·min-1纯氧,小鼠翻正反射恢复20 min后将所有小鼠放回其各自笼中。
1.5 免疫荧光双染色检测小鼠海马神经元凋亡率每组随机选取6只小鼠,分别于麻醉后6和24 h腹腔注射氯胺酮(10 g·L-1)和甲苯噻嗪(1 g·L-1)混合液(0.01 mL·g-1体质量),麻醉后暴露心脏,用冰冷的磷酸盐缓冲液行穿心灌注,直至肝脏颜色变白后迅速取出鼠脑,沿中线将其分为左、右两部分。左半脑组织先于干冰中冷冻5 min,于-80℃储存。右半脑组织用4%多聚甲醛固定过夜,行石蜡包埋和切片(4 μm)。切片经抗NeuN抗体染色后,根据试剂盒说明书行TUNEL染色,DAPI覆盖切片,应用光学显微镜观察海马神经元凋亡情况。细胞凋亡率=凋亡细胞数/(凋亡细胞数+正常细胞数)×100%。
1.6 Western blotting法检测小鼠脑组织中Aβ、Aβ40、Aβ42、Cleaved caspase-3、Bax和Bcl-2蛋白表达水平将小鼠左半脑海马组织从-80℃冰箱中取出,取0.2 g置入EP管中,加入冰冷的RIPA裂解液,4℃、8000 g离心5 min,取其上清。蛋白质样本依据待测蛋白质的相对分子质量,由12%SDS-PAGE分离并应用半干式电转移器(45 V, 4℃过夜, Amersham TE 70)将其转移至PVDF膜上,加封闭液后在摇床上室温振荡1 h,再加入特异性一抗,4℃冰箱过夜,而后进行洗膜和二抗孵育。采用凝胶成像和免疫印迹检测系统(WEALTEC公司, 美国)测量条带吸光度(A)值,以目的蛋白条带A值与β-actin条带A值的比值表示目的蛋白表达水平。
1.7 统计学分析采用SPSS 22.0统计软件进行统计学处理。小鼠血清和脑组织中锌水平、海马神经元凋亡率及海马组织中Aβ、Cleaved caspase-3表达水平和Bax/Bcl-2比值均以x±s表示,2组间样本均数比较采用t检验,多组间样本均数比较采用双因素方差分析。以P < 0.05表示差异有统计学意义。
2 结果 2.1 各组APP/PS1转基因小鼠血清锌和脑锌检测结果造模前各组小鼠血清锌水平比较差异无统计学意义(P>0.05)。与常锌组比较,10月龄缺锌组和缺锌+异氟醚组小鼠血清锌和脑组织中锌水平明显降低(P < 0.05或P < 0.01),表明成功建立缺锌APP/PS1转基因小鼠模型。见表 1和2。
| [n=24, x±s, ρB/(μg·L-1)] | ||
| Group | Serum of Zn | |
| Before modeling | After modeling | |
| ZA | 1 219.47±238.49 | 1 185.15±185.25 |
| ZA+Iso | 1 255.24±302.78 | 1 228.51±258.82 |
| ZD | 1 277.85±290.44 | 662.42±237.45* |
| ZD+Iso | 1 238.95±315.65 | 613.93±196.66* |
| * P < 0.01 compared with ZA group. | ||
| [n=6, x±s, wB/(μg·g-1)] | ||
| Group | Zn | |
| Cortex | Hippocampus | |
| ZA | 17.69±3.42 | 16.87±2.62 |
| ZA+Iso | 17.22±2.87 | 16.43±1.95 |
| ZD | 10.44±1.75** | 9.75±2.17** |
| ZD+Iso | 11.12±2.33* | 10.06±1.83** |
| * P < 0.05, * * P < 0.01 compared with ZA group. | ||
TUNEL染色阳性代表各种神经细胞的凋亡(包括神经元细胞);NeuN染色阳性代表神经元细胞的凋亡,当NeuN染色阳性细胞增多,则表示凋亡的神经元细胞数量的增加。与常锌组比较,常锌+异氟醚组小鼠接受异氟醚麻醉后6 h海马神经元凋亡率增高(P < 0.05),麻醉后24 h海马神经元凋亡率无明显变化(P>0.05)。与常锌组比较,缺锌组小鼠海马神经元凋亡率增高(P < 0.01),缺锌+异氟醚组小鼠接受异氟醚麻醉后6和24 h海马神经元凋亡率进一步增高(P < 0.01)。与缺锌组比较,缺锌+异氟醚组小鼠接受异氟醚麻醉后6和24 h海马神经元凋亡率增高(P < 0.05)。见图 1(插页一)和表 3。
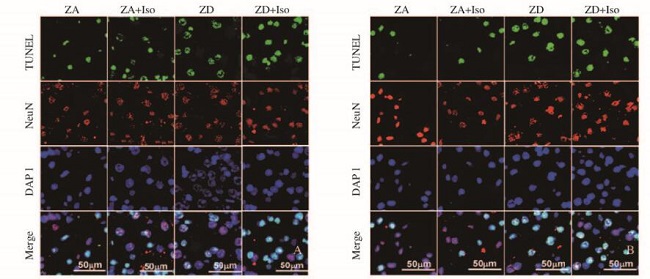
|
| A: 6 h after isoflurane exposure; B: 24 h after isoflurane exposure. 图 1 异氟醚麻醉后各组小鼠海马CA1区神经元凋亡情况 Figure 1 Apoptosis of neurons in hippocampal CA1 region after isoflurane exposure of mice in various groups |
|
|
| (n=6, x±s, η/%) | ||
| Group | Apoptotic rate | |
| 6 h after isoflurane | 24 h after isoflurane | |
| ZA | 30.3±3.4 | 30.6±3.7 |
| ZA+Iso | 39.2±4.5* | 33.3±4.2 |
| ZD | 43.2±5.6** | 43.6±4.9** |
| ZD+Iso | 53.4±6.7**△ | 54.7±7.2**△ |
| * P < 0.05,* * P < 0.01 compared with ZA group;△ P < 0.05 compared with ZD group. | ||
与常锌组比较,常锌+异氟醚组小鼠海马组织中总Aβ、Aβ40和Aβ42蛋白表达水平均无明显变化(P>0.05),缺锌组小鼠海马组织中总Aβ和Aβ40蛋白表达水平无明显变化(P>0.05),而Aβ42蛋白表达水平升高(P < 0.05);缺锌+异氟醚组小鼠海马组织中Aβ和Aβ40蛋白表达水平无明显变化(P>0.05),Aβ42蛋白表达水平进一步升高(P < 0.01)。与缺锌组比较,缺锌+异氟醚组小鼠海马组织中Aβ42蛋白表达水平升高(P < 0.05)。见图 2和表 4。
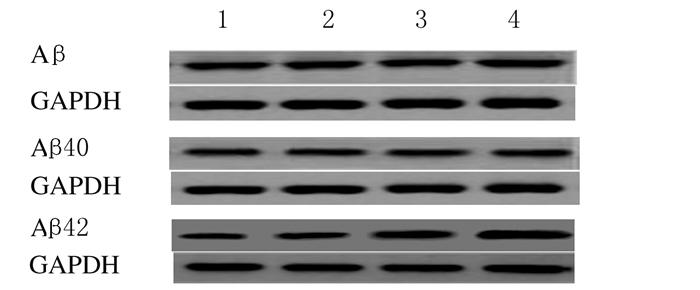
|
| Lane 1:ZA group; Lane 2:ZA+Iso group; Lane 3:ZD group; Lane 4:ZD+Iso group. 图 2 各组小鼠海马组织中总Aβ、Aβ40和Aβ42蛋白表达电泳图 Figure 2 Electrophoregram of expressions of Aβ, Aβ40and Aβ42 in hippocampus of mice in various groups |
|
|
| (n=6, x±s) | |||
| Group | Aβ/GAPDH | Aβ40/GAPDH | Aβ42/GAPDH |
| ZA | 0.66±0.05 | 0.39±0.03 | 0.36±0.05 |
| ZA+Iso | 0.68±0.07 | 0.43±0.07 | 0.42±0.07 |
| ZD | 0.65±0.06 | 0.40±0.05 | 0.87±0.10* |
| ZD+Iso | 0.71±0.08 | 0.42±0.06 | 1.2±0.12**△ |
| * P < 0.05, * * P < 0.01 compared with ZA group;△ P < 0.05 compared with ZD group. | |||
与常锌组比较,常锌+异氟醚组、缺锌组和缺锌+异氟醚组小鼠海马组织中Cleaved caspase-3蛋白表达水平和Bax/Bcl-2比值均增加(P < 0.05或P < 0.01)。与缺锌组比较,缺锌+异氟醚组小鼠海马Cleaved caspase-3蛋白表达水平和Bax/Bcl-2比值明显增加(P < 0.05或P < 0.01)。见图 3、4和表 5。
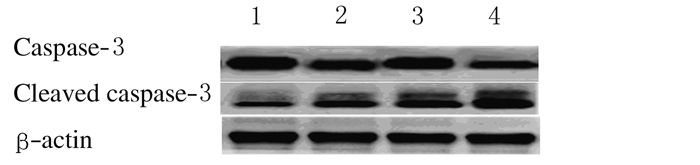
|
| Lane 1:ZA group; Lane 2:ZA+Iso group; Lane 3:ZD group; Lane 4:ZD+Iso group. 图 3 各组小鼠海马CA1区Cleaved caspase-3蛋白表达电泳图 Figure 3 Electrophoregram of expressions of Cleaved caspase-3 in hippocampal CA1 region of mice in various groups |
|
|
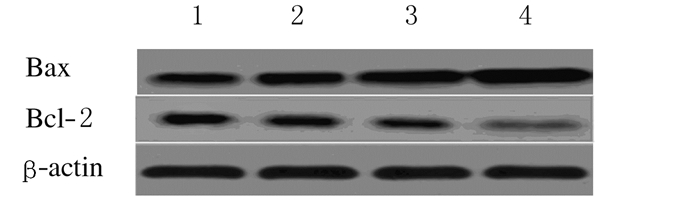
|
| Lane 1:ZA group; Lane 2:ZA+Iso group; Lane 3:ZD group; Lane 4:ZD+Iso group. 图 4 各组小鼠海马CA1区Bax和Bcl-2蛋白表达电泳图 Figure 4 Electrophoregram of expressions of Bax and Bcl-2 in hippocampal CA1 region of mice in various groups |
|
|
| (n=6, x±s) | ||
| Group | Cleaved caspase-3/β-actin | Bax/Bcl-2 |
| ZA | 0.32±0.05 | 0.78±0.15 |
| ZA+Iso | 0.63±0.07* | 1.71±0.25** |
| ZD | 0.82±0.08** | 5.69±0.40** |
| ZD+Iso | 1.22±0.11**△△ | 11.50±0.50**△ |
| * P < 0.05, * * P < 0.01 compared with ZA group;△ P < 0.05,△△ P < 0.01 compared with ZD group. | ||
APP/PS1转基因小鼠是目前应用最广泛的AD小鼠模型之一,6月龄开始出现海马区Aβ大量沉积,10月龄APP/PS1转基因小鼠处于AD临床前期。吸入麻醉药异氟醚是目前临床最常用全身麻醉药之一,常用浓度为1.0%~2.0%。因此,本研究选择10月龄APP/PS1转基因小鼠暴露于1.4%异氟醚2 h,观察异氟醚麻醉对临床前期AD海马神经元凋亡的影响。
AD发病机制复杂,病因尚不十分清楚,其主要神经病理表现为Aβ聚集、Tau蛋白过度磷酸化和神经元凋亡等。研究[1, 16-17]表明:全射麻醉能够加重AD的神经病理改变。研究[1-3, 16]显示:常用吸入麻醉药异氟醚具有与AD密切相关的神经毒性,能够促进Aβ沉积和Tau蛋白过度磷酸化,加剧AD神经元凋亡等神经病理进程。然而,临床常用浓度异氟醚麻醉是否加重临床前期AD患者神经病理进程及其相关影响因素,尚需进一步研究。
金属元素锌是机体重要的必需微量元素之一,广泛分布于中枢神经系统,参与多种蛋白的合成和信号通路的转导,发挥重要生物学效应。研究[17-18]显示:缺锌加剧AD动物以及AD患者的Aβ沉积和细胞凋亡等神经病理进程。调查[15, 19]显示:AD患者发生缺锌风险高,全球近25%AD患者伴有不同程度的锌缺乏。研究[20]显示:锌缺乏能够促进海马神经元凋亡,影响AD神经病理进程。本研究结果显示:与常锌组比较,缺锌组小鼠海马神经元凋亡率增加,缺锌+异氟醚组小鼠海马神经元凋亡率进一步增加,表明缺锌不仅能够诱导海马神经元凋亡,还能够加剧异氟醚麻醉AD小鼠的神经病理进程,进一步加重海马神经元凋亡。
AD等神经系统变性疾病中神经细胞的最终结局为细胞凋亡。AD时脑内Aβ产生和清除机制失平衡,Aβ大量沉积,通过细胞凋亡等途径导致神经元死亡,引起神经系统退行性变[2, 21]。研究[3, 22-26]显示:暴露异氟醚能够增加细胞Aβ聚集,进而导致细胞凋亡的发生;吸入麻醉药异氟醚对Aβ聚集和神经元凋亡的影响与异氟醚暴露浓度、时间、次数以及动物年龄等多种因素相关;应用1%最低肺泡有效浓度(MAC)为1的异氟醚麻醉12月龄APP695小鼠4 h可导致小鼠Aβ病理性聚集及海马神经元损伤。研究[2]显示:应用2%的异氟醚麻醉7月龄Tg2576小鼠(麻醉20 min,2次/周),可导致10月龄小鼠海马组织Aβ表达水平明显增加。然而,Xu等[27]研究显示:0.5%异氟醚麻醉30 min对AD小鼠神经元凋亡无影响。本研究结果显示:与常锌组比较,常锌+异氟醚组小鼠异氟醚麻醉后6 h海马神经元凋亡率增高,麻醉后24 h海马神经元凋亡率无明显变化,表明1.4%异氟醚单次、短时间麻醉能够短暂加重常锌APP/PS1转基因小鼠海马神经元凋亡。本研究结果还显示:与缺锌组比较,缺锌+异氟醚组小鼠麻醉后6和24 h海马神经元凋亡率和Aβ42表达均明显增高,表明缺锌可能是吸入麻醉药异氟醚诱导AD发病的一个重要风险因素,能够加剧异氟醚对AD的神经毒性作用,促进细胞凋亡。
细胞凋亡的机制尚不十分清楚,可能与线粒体损伤、氧化应激和钙稳态失衡等多种因素有关联。Caspase和Bcl-2家族在线粒体细胞凋亡通路中发挥着重要作用。Caspase-3具有促进细胞凋亡的作用,是Caspase级联反应中凋亡的最终实施因子。Bcl-2家族包括促凋亡因子Bax和抗凋亡因子Bcl-2,Bax能与Bcl-2形成异二聚体,对Bcl-2产生抑制作用,Bax/Bcl-2比值是决定细胞凋亡发生的关键指标[14]。AD时Aβ聚集,能够增强Bax表达,引起Bax/Bcl-2比值升高,激活Caspase-3,最终导致细胞凋亡[28]。本研究结果显示:与常锌组比较,缺锌组小鼠海马神经元凋亡率、Bax/Bcl-2比值、Aβ42和Cleaved caspase-3表达水平均升高;而与缺锌组比较,缺锌+异氟醚组小鼠海马神经元凋亡率、Bax/Bcl-2比值、Aβ42和Cleaved caspase-3表达水平均进一步增加,表明缺锌能够加重异氟醚对AD神经元损伤,加剧Aβ聚集,激活Bax,抑制Bcl-2,引起Bax/Bcl-2比值增加,促进Caspase活化,诱导神经元凋亡。
综上所述,10月龄常锌APP/PS1转基因小鼠给予1.4%异氟醚麻醉2 h能够短暂加重其海马神经元凋亡;1.4%异氟醚麻醉2 h能够明显加重缺锌APP/PS1转基因小鼠海马神经元凋亡,其机制可能与促进海马Aβ42聚集、增强Bax表达、抑制Bcl-2表达及激活Caspase-3有关。
| [1] | Jiang J, Jiang H. Effect of the inhaled anesthetics isoflurane, sevoflurane and desflurane on the neuropathogenesis of Alzheimer' s disease[J]. Mol Med Rep, 2015, 12(1): 3–12. DOI:10.3892/mmr.2015.3424 |
| [2] | Perucho J, Rubio I, Casarejos MJ, et al. Anesthesia with isoflurane increases amyloid pathology in mice models of Alzheimer' s disease[J]. J Alzheimers Dis, 2010, 19(4): 1245–1257. DOI:10.3233/JAD-2010-1318 |
| [3] | Li C, Liu S, Xing Y, et al. The role of hippocampal tau protein phosphorylation in isoflurane-induced cognitive dysfunction in transgenic APP695 mice[J]. Anesth Analg, 2014, 119(2): 413–419. DOI:10.1213/ANE.0000000000000315 |
| [4] | Marger L, Schubert CR, Bertrand D. Zinc:an underappreciated modulatory factor of brain function[J]. Biochem Pharmacol, 2014, 91(4): 426–435. DOI:10.1016/j.bcp.2014.08.002 |
| [5] | Myers S, Shastri MD, Adulcikas J, et al. Zinc and gastrointestinal disorders:A role for the zinc transporters zips and ZnTs[J]. Curr Pharm Des, 2017, 23(16): 2328–2332. |
| [6] | Prakash A, Bharti K, Majeed AB. Zinc:indications in brain disorders[J]. Fundam Clin Pharmacol, 2015, 29(2): 131–149. DOI:10.1111/fcp.2015.29.issue-2 |
| [7] | McAllister BB, Dyck RH. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function[J]. Neurosci Biobehav Rev, 2017, 80: 329–350. DOI:10.1016/j.neubiorev.2017.06.006 |
| [8] | Brewer GJ, Kaur S. Zinc deficiency and zinc therapy efficacy with reduction of serum free copper in Alzheimer' s disease[J]. Int J Alzheimers Dis, 2013, 2013: 586365. |
| [9] | Tyszka-Czochara M, Grzywacz A, Gdula-Argasińska J, et al. The role of zinc in the pathogenesis and treatment of central nervous system (CNS) diseases.Implications of zinc homeostasis for proper CNS function[J]. Acta Pol Pharm, 2014, 71(3): 369–377. |
| [10] | Brewer GJ. Divalent copper as a major triggering agent in Alzheimer' s disease[J]. Alzheimers Dis, 2015, 46(3): 593–604. DOI:10.3233/JAD-143123 |
| [11] | Brewer G. Copper-2 ingestion, plus increased meat eating leading to increased copper absorption, are major factors behind the current epidemic of Alzheimer' s disease[J]. Nutrients, 2015, 7(12): 10053–10064. DOI:10.3390/nu7125513 |
| [12] | Wang ZX, Tan L, Wang HF, et al. Serum iron, zinc, and copper levels in patients with Alzheimer' s disease:A replication study and meta-analyses[J]. J Alzheimers Dis, 2015, 47(3): 565–581. DOI:10.3233/JAD-143108 |
| [13] | Ventriglia M, Brewer GJ, Simonelli I, et al. Zinc in Alzheimer' s Disease:A Meta-Analysis of Serum, Plasma, and Cerebrospinal Fluid Studies[J]. J Alzheimers Dis, 2015, 46(1): 75–87. DOI:10.3233/JAD-141296 |
| [14] | Paz-Tal O, Canfi A, Marko R, et al. Effect of changes in food groups intake on magnesium, Zinc, copper, and selenium serum levels during 2 years of dietary intervention[J]. J Am Coll Nutr, 2015, 34(1): 1–14. DOI:10.1080/07315724.2013.875432 |
| [15] | Vural H, Demerin H, Kara Y, et al. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer' s disease[J]. J Trace Elem Med Biol, 2010, 24(3): 169–173. DOI:10.1016/j.jtemb.2010.02.002 |
| [16] | Dong Y, Wu X, Xu Z, et al. Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Aβ generation[J]. PLoS One, 2012, 7(6): e39386. DOI:10.1371/journal.pone.0039386 |
| [17] | Pang W, Leng X, Lu H, et al. Depletion of intracellular zinc induces apoptosis of cultured hippocampal neurons through suppression of ERK signaling pathway and activation of caspase-3[J]. Neurosci Lett, 2013, 552(27): 140–145. |
| [18] | SzewczykB. Zinc homeostasis and neurodegenerative disorders[J]. Front Aging Neurosci, 2013, 5: 33. |
| [19] | Ventriglia M, Brewer GJ, Simonelli I, et al. Zinc in Alzheimer' s disease:A Meta-analysis of serum, plasma, and cerebrospinal fluid studies[J]. J Alzheimers Dis, 2015, 46(1): 75–87. DOI:10.3233/JAD-141296 |
| [20] | Adamo AM, Oteiza PI. Zinc deficiency and neurodevelopment:The case of neurons[J]. Biofactors, 2010, 36(2): 117–124. |
| [21] | Zurek AA, Yu J, Wang DS, et al. Sustained increase in α5GABAA receptor function impairs memory after anesthesia[J]. J Clin Invest, 2014, 124(12): 5437–5441. DOI:10.1172/JCI76669 |
| [22] | Li XY, Xu L, Liu CL, et al. Electroacupuncture intervention inhibits the decline of learning-memory ability and overexpression of cleaved caspase-3 and bax in hippocampus induced by isoflurane in APPswe/PS 1[J]. Zhen Ci Yan Jiu, 2016, 41(1): 24–30. |
| [23] | Yu J, Luo X, Xu H, et al. Identification of the key molecules involved in chronic copper exposure-aggravated memory impairment in transgenic mice of Alzheimer' s disease using proteomic analysis[J]. J Alzheimers Dis, 2015, 44(2): 455–469. |
| [24] | Huang H, Wang L, Cao M, et al. Isolation housing exacerbates Alzheimer' s disease-like pathophysiology in aged APP/PS1 mice[J]. Int J Neuropsychopharmacol, 2015, 18(7): u116. DOI:10.1093/ijnp/pyu116 |
| [25] | Feng C, Liu Y, Yuan Y, et al. Isoflurane anesthesia exacerbates learning and memory impairment in zinc-deficient APP/PS1 transgenic mice[J]. Neuropharmacology, 2016, 111: 119–129. DOI:10.1016/j.neuropharm.2016.08.035 |
| [26] | Bao Y, Zhang Y, Chu J, et al. Effects of grain-sized moxibustion on expression of Abeta(1-42) in prefrontal cortex and hippocampus in double-transgenic AD mice[J]. Zhongguo Zhen Jiu, 2015, 35(1): 59–65. |
| [27] | Xu Z, Dong Y, Wu X, et al. The potential dual effects of anesthetic isoflurane on Aβ-induced apoptosis[J]. Curr Alzheimer Res, 2011, 8(7): 741–752. DOI:10.2174/156720511797633223 |
| [28] | Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK:a requisite gateway to mitochondrial dysfunction and death[J]. Science, 2001, 292(5517): 727–730. DOI:10.1126/science.1059108 |
 2018, Vol. 44
2018, Vol. 44


