扩展功能
文章信息
- 赵治艳, 盛今东, 刘宝国
- ZHAO Zhiyan, SHENG Jindong, LIU Baoguo
- 恶性甲状腺肿物和甲状腺乳头状癌Ⅵ区淋巴结转移的危险因素
- Risk factors of malignant thyroid neoplasms and papillary thyroid cancer level Ⅵ lymph node metastasis
- 吉林大学学报(医学版), 2017, 43(02): 334-338
- Journal of Jilin University (Medicine Edition), 2017, 43(02): 334-338
- 10.13481/j.1671-587x.20170223
-
文章历史
- 收稿日期: 2016-07-06
2. 航天中心医院普外科, 北京 100039
2. Department of General Surgery, Aerospace Central Hospital, Beijing 100039, China
近年来,甲状腺乳头状癌 (papillary thyroid cancer,PTC) 的发病率逐年上升,尤其在女性患者中上升更为明显[1-2]。有研究[3]报道:微小钙化可作为甲状腺乳头状癌的危险因素,但目前尚不能明确与淋巴结转移的关系。PTC的治疗方法很多,包括手术、放射碘、放疗及生物治疗等,其中手术治疗是其主要治疗方法[2-4]。由于患者个体差异、肿瘤及治疗等因素会影响PTC的复发和患者生存期,因此手术治疗面临的挑战就是平衡手术的受益和风险,为患者选择最适合的治疗方案[3-5]。本研究着手于患者个体差异及肿瘤因素,评估肿瘤转移风险,为PTC的治疗及预后提供指导。目前国内外学者[4, 6]对PTC手术方式的选择及其淋巴结的清扫范围有很大争议,能否准确判断PTC的淋巴结转移情况成了目前亟待解决的问题。本研究对99例PTC病例及22例结节性甲状腺肿病例的临床资料进行回顾性分析,从影像学角度分析甲状腺肿物的良恶性与PTC患者Ⅵ区淋巴结转移的危险因素,并探讨这些因素指导临床治疗及预后的意义。
1 资料与方法 1.1 临床资料患者的病历资料均来北京大学肿瘤医院头颈外科,患者已签署知情同意书。从2011年3月—2013年9月的病例中,筛选出99例PTC患者行甲状腺癌根治术并行Ⅵ区淋巴结清扫术及22例结节性甲状腺肿患者行甲状腺腺叶切除术病例。其中,男性28例,女性93例,年龄为15~69岁;平均年龄为 (42.8±10.9) 岁;按照2010年美国癌症联合委员会 (AJCC)/国际抗癌联合会 (UICC) TNM分期标准,病例中T1N0M0期50例,T1N1aM0期36例,T2N0M0期1例,T2N1aM0期5例,T3N1aM0期4例,T4aN1aM0期3例。所有患者随访至2016年3月均未见复发。
1.2 手术范围PTC患者均行甲状腺癌根治术并行Ⅵ区淋巴结清扫术,Ⅵ区清扫范围上界为舌骨水平,两侧至颈总动脉内侧缘,下界至颈总动脉根部。
1.3 观察方法及指标排除病理中未明确报告PTC及淋巴结转移情况的病例,依据病理结果将99例PTC患者分为2组,Ⅵ区淋巴结转移组48例和未转移组51例。分析病历资料中性别、年龄、肿瘤大小、B超显示钙化及边界情况等临床因素。
1.4 统计学分析应用SPSS 19.0统计软件进行统计学分析。采用Pearson χ2检验统计方法分析性别、年龄、B超显示钙化及边界情况与甲状腺肿物良恶性的相关性。并利用Pearson χ2检验分析B超提示同时存在钙化及边界不清与PTC患者Ⅵ区淋巴结转移的相关性。
2 结果 2.1 患者年龄、B超显示钙化和边界不清与甲状腺肿物良恶性的关系在PTC患者和结节性甲状腺肿患者中,性别分布差异无统计学意义 (图 1A)。在PTC患者中,62.63%(62/99) 为年龄≤45岁,而在结节性甲状腺肿患者中,只有36.36%(8/22) 为年龄≤45岁 (图 1B)。B超结果提示:79.80%(79/99) 的PTC患者存在钙化,只有31.82%(7/22) 的结节性甲状腺肿患者存在钙化 (图 1C);80.81%(80/99) 的PTC患者存在肿物边界不清,只有18.18%(4/99) 的结节性甲状腺肿患者存在肿物边界不清 (图 1D)。相关性分析结果显示:与结节性甲状腺肿比较,性别与PTC无相关性 (P=0.959, OR=0.972);但年龄与其有相关性 (P < 0.05, OR=0.341),且B超显示钙化 (P < 0.001, OR=0.115) 及边界不清 (P < 0.001, OR=18.947) 与PTC有相关性,见表 1。
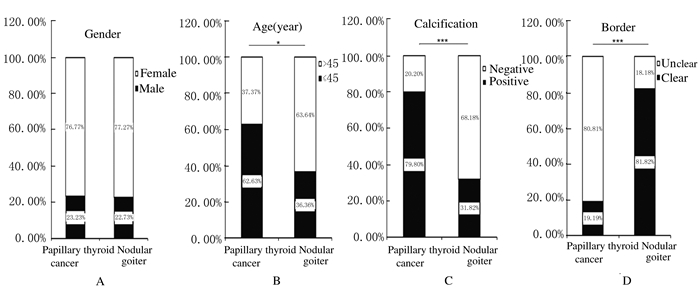
|
| A: Gender; B: Age (≤45 years old and > 45 years old, P < 0.05); C: Calcification (P < 0.001); D: Border (P < 0.001). 图 1 各临床因素在99例PTC和22例结节性甲状腺肿患者中的分布 Figure 1 Distribution of clinical factors in 99 patients with PTC and 22 patients with nodular goiter |
|
|
| Clinical factor | PTC (n=99) | Nodular goiter (n=22) | χ2 | P | OR |
| Gender | |||||
| Male Female |
23 76 |
5 17 |
0.003 | 0.959 | 0.972 |
| Age (year) | |||||
| ≤45 >45 |
62 37 |
8 14 |
5.092 | 0.024 | 0.341 |
| Calcification | |||||
| Positive Negative |
79 20 |
7 15 |
20.156 | < 0.001 | 0.115 |
| Border | |||||
| Clear Unclear |
19 80 |
18 4 |
33.256 | < 0.001 | 18.947 |
存在和不存在Ⅵ区淋巴结转移的PTC患者中,性别、B超中钙化和边界分布差异不明显。但在淋巴结转移组中,77.08%(37/48) 为年龄≤45岁的患者;在淋巴结未转移组中,只有49.02%(25/51) 为年龄≤45岁的患者;B超结果显示:77.08%(37/48) 淋巴结转移的PTC患者为>1 cm的癌灶,只有37.25%(19/51) 的淋巴结未转移的PTC患者为>1 cm的癌灶。见图 2。性别 (P=0.379, OR=1.523)、B超显示钙化 (P=0.064, OR=2.649) 和边界不清 (P=0.536, OR=0.727) 与PTC患者Ⅵ区淋巴结转移无相关性。但患者年龄与PTC患者Ⅵ区淋巴结转移有相关性 (P < 0.01, OR=3.498);且肿瘤大小与PTC患者Ⅵ区淋巴结转移有相关性 (P < 0.001, OR=0.177)。见表 2。
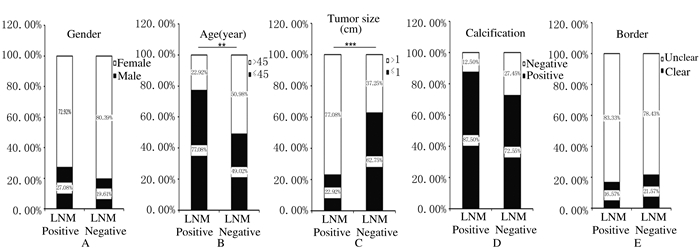
|
| A: Gender; B: Age (≤45 years old and > 45 years old, P < 0.01); C: Tumor size (≤1 cm and > 1cm, P < 0.001); D: Calcification; E: Border. 图 2 各临床因素在存在和不存在Ⅵ区淋巴结转移PTC患者中的分布 Figure 2 Distribution of clinical factors in PTC patients with and without level Ⅵ lymph node metastasis |
|
|
| Clinical factor | Lymph node metastasis (LNM) | χ2 | P | OR | |
| Positive (n=48) | Negative (n=51) | ||||
| Gender | |||||
| Male Female |
13 35 |
10 41 |
0.775 | 0.379 | 1.523 |
| Age (year) | |||||
| ≤45 >45 |
37 11 |
25 26 |
8.320 | 0.004 | 3.498 |
| Tumor size (cm) | |||||
| ≤1 >1 |
11 37 |
32 19 |
15.965 | < 0.001 | 0.177 |
| Calcification | |||||
| Positive Negative |
42 6 |
37 14 |
3.429 | 0.064 | 2.649 |
| Border | |||||
| Clear Unclear |
8 40 |
11 40 |
0.383 | 0.536 | 0.727 |
B超显示同时存在钙化和边界不清与PTC患者Ⅵ区淋巴结转移不存在明显的相关性 (P=0.189, OR=1.781)。见表 3。
| Clinical factor | Lymph node metastasis | χ2 | P | OR | |
| Positive (n=48) | Negative (n=51) | ||||
| Calcification and unclear border Others |
36 12 |
32 19 |
1.727 | 0.189 | 1.781 |
甲状腺癌日渐上升的发病率已引起许多学者的关注,如何为患者选择最合适的治疗方案主要取决于医生对甲状腺疾病的诊断经验[3, 6-8]。由于PTC的不同手术引起的并发症及对患者生活质量的影响程度不同,所以目前PTC的手术方式及手术范围仍存在很大争议。支持为PTC患者行常规性及预防性颈中央淋巴结清扫术的学者[9-13]认为颈部中央区的淋巴结转移非常常见,区域淋巴结转移与疾病的复发明显相关,且病情反复发作的患者有较高的死亡率。然而,不支持行常规性及预防性颈中央淋巴结清扫术的学者[10, 14-15]认为区域淋巴结转移对总生存率无影响而且会增加并发症的风险,包括喉返神经和甲状旁腺的损伤等。所以,准确判断患者病情及淋巴结转移情况并为患者选择最合适的治疗方案非常必要。
目前越来越多的年轻患者罹患PTC,且PTC的淋巴结转移也好发于年轻患者。有研究[16-17]表明PTC及淋巴结转移与瘤体钙化有关。为进一步探讨甲状腺肿物的影像学资料与其良恶性及Ⅵ区淋巴结转移的关系,本文作者从性别、年龄、钙化及边界等方面进行相关性分析发现:PTC较多发生于年轻患者,并且容易发生淋巴结转移。甲状腺肿物B超显示存在有钙化或边界不清时,肿物倾向于恶性;但是尚不能说明存在淋巴结转移。即使同时存在钙化及边界不清,也不能因此判断其转移的概率增加。除此之外,与其他学者[18-19]的研究结果一致,本研究还发现肿瘤大小与PTC的淋巴结转移明显相关,肿瘤直径>1cm的PTC容易发生淋巴结转移。这就说明可以通过年龄和影像学资料等综合考虑,初步判断甲状腺肿物的良恶性以及PTC的淋巴结转移情况,从而为患者选择最合适的治疗方案并判断预后。
综上所述,年龄可以作为恶性甲状腺肿物和PTC患者Ⅵ区淋巴结转移的危险因素。B超显示存在钙化和边界不清是判断甲状腺肿物良恶性的危险因素,但尚不能提示是否有Ⅵ区淋巴结转移。此外,肿瘤大小在一定程度上可判断PTC的Ⅵ淋巴结转移。是否行Ⅵ区淋巴结清扫术,需在术中观察肿瘤大小、性状及淋巴结情况进一步确定。本研究仍存在一些不足,受各种条件限制及肿瘤分期影响,本文作者将进一步收集更多病例资料,从多因素角度分析研究PTC及淋巴结转移的危险因素。
| [1] | Jemal A, Bray F, Center MM, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61(2): 69–90. DOI:10.3322/caac.v61:2 |
| [2] | Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53, 856 cases of thyroid carcinoma treated in the U.S., 1985-1995[J]. Cancer, 1998, 83(12): 2638–2648. DOI:10.1002/(ISSN)1097-0142 |
| [3] | Elaraj DM, Sturgeon C. Adequate surgery for papillary thyroid cancer[J]. Surgeon, 2009, 7(5): 286–289. DOI:10.1016/S1479-666X(09)80006-1 |
| [4] | Carling T, Udelsman R. Thyroid cancer[J]. Annu Rev Med, 2014, 65: 125–137. DOI:10.1146/annurev-med-061512-105739 |
| [5] | Nixon IJ, Ganly I, Shah JP. Thyroid cancer:surgery for the primary tumor[J]. Oral Oncol, 2013, 49(7): 654–658. DOI:10.1016/j.oraloncology.2013.03.439 |
| [6] | Sakorafas GH, Sampanis D, Safioleas M. Cervical lymph node dissection in papillary thyroid cancer:current trends, persisting controversies, and unclarified uncertainties[J]. Surg Oncol, 2010, 19(2): e57–70. DOI:10.1016/j.suronc.2009.04.002 |
| [7] | Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002[J]. JAMA, 2006, 295(18): 2164–2167. DOI:10.1001/jama.295.18.2164 |
| [8] | Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer[J]. Ann Surg, 2007, 246(3): 375–381. DOI:10.1097/SLA.0b013e31814697d9 |
| [9] | Shindo M, Wu JC, Park EE, et al. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer[J]. Arch Otolaryngol Head Neck Surg, 2006, 132(6): 650–654. DOI:10.1001/archotol.132.6.650 |
| [10] | Ito Y, Jikuzono T, Higashiyama T, et al. Clinical significance of lymph node metastasis of thyroid papillary carcinoma located in one lobe[J]. World J Surg, 2006, 30(10): 1821–1828. DOI:10.1007/s00268-006-0211-5 |
| [11] | Pereira JA, Jimeno J, Miquel J, et al. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma[J]. Surgery, 2005, 138(6): 1095–1100. DOI:10.1016/j.surg.2005.09.013 |
| [12] | Mazzaferri EL, Kloos RT. Clinical review 128:Current approaches to primary therapy for papillary and follicular thyroid cancer[J]. J Clin Endocrinol Metab, 2001, 86(4): 1447–1463. DOI:10.1210/jcem.86.4.7407 |
| [13] | Harwood J, Clark OH, Dunphy JE. Significance of lymph node metastasis in differentiated thyroid cancer[J]. Am J Surg, 1978, 136(1): 107–112. DOI:10.1016/0002-9610(78)90209-X |
| [14] | Toniato A, Boschin I, Casara D, et al. Papillary thyroid carcinoma:factors influencing recurrence and survival[J]. Ann Surg Oncol, 2008, 15(5): 1518–1522. DOI:10.1245/s10434-008-9859-4 |
| [15] | Sywak M, Pasieka JL, Ogilvie T. A review of thyroid cancer with intermediate differentiation[J]. J Surg Oncol, 2004, 86(1): 44–54. DOI:10.1002/(ISSN)1096-9098 |
| [16] | Ye ZQ, Gu DN, Hu HY, et al. Hashimoto's thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer[J]. World J Surg Oncol, 2013, 11(1): 1–7. DOI:10.1186/1477-7819-11-1 |
| [17] | Bai Y, Zhou G, Nakamura M, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma[J]. Mod Pathol, 2009, 22(7): 887–894. DOI:10.1038/modpathol.2009.38 |
| [18] | Ito Y, Fukushima M, Kihara M, et al. Investigation of the prognosis of patients with papillary thyroid carcinoma by tumor size[J]. Endocr J, 2012, 59(6): 457–464. DOI:10.1507/endocrj.EJ12-0013 |
| [19] | Liu X, Zhu L, Wang Z, et al. Evolutionary features of thyroid cancer in patients with thyroidectomies from 2008 to 2013 in China[J]. Sci Rep, 2016, 6: 28414. DOI:10.1038/srep28414 |
 2017, Vol. 43
2017, Vol. 43


