扩展功能
文章信息
- 霍连广, 严庆涛, 严晶月, 李娜, 苏寒, 张美家, 毛淑梅, 高志芹, 曲梅花
- HUO Lianguang, YAN Qingtao, YAN Jingyue, LI Na, SU Han, ZHANG Meijia, MAO Shumei, GAO Zhiqin, QU Meihua
- 十二指肠空肠旁路术对ZDF大鼠BP肠袢部位炎症状态的改善作用及其机制
- Improvement effect of duodenal-jejunal bypass on inflammatory status of biliopancreatic limb of ZDF rats and its mechanism
- 吉林大学学报(医学版), 2017, 43(06): 1155-1160
- Journal of Jilin University (Medicine Edition), 2017, 43(06): 1155-1160
- 10.13481/j.1671-587x.20170616
-
文章历史
- 收稿日期: 2017-06-20
2. 山东省潍坊市人民医院 小儿外科, 山东 潍坊 261041
2. Department of Pediatric Surgery, Weifang People's Hospital, Shandong Province, Weifang 261041, China
2型糖尿病(type 2 diabetes,T2DM)是严重威胁人类健康的慢性非感染性疾病,近年来发病率有急剧上升的趋势[1]。研究[2]显示:代谢性手术在治疗肥胖症的同时常伴有T2DM的长期缓解,具有不依赖于体质量下降的抗糖尿病作用,已成为治疗肥胖型T2DM的重要手段。代谢手术治疗T2DM的机制尚未完全阐明。十二指肠空肠旷置术(duodenal-jejunal bypass, DJB)是Rubino等[3-4]设计的为研究代谢手术抗糖尿病机制的实验型手术方式。T2DM的主要原因是胰岛素抵抗及胰岛细胞功能障碍。近年来,炎症假说备受关注,尤其是炎症在胰岛素抵抗中的作用[5]。研究[6]表明T2DM是一种自然免疫与低度炎症性疾病。大量临床流行病学研究[7-8]显示:T2DM的发病与炎症因子存在密切联系。研究[9]显示:DJB通过降低T2DM大鼠肝脏和脂肪部位炎症相关的JNK信号通路活性,使血糖水平得到明显的改善;研究[10]显示DJB还可以通过降低体内炎症因子的表达从而改善T2DM。ZDF大鼠为高脂、高糖、高胰岛素血症的肥胖型T2DM大鼠模型[11]。本研究通过建立DJB干预ZDF大鼠模型,检测大鼠血糖稳态及手术后旷置段(又称十二指肠和近端空肠部位,BP段,biliopancreatic limb,胆胰段)关键分子AMPK磷酸化程度的变化以及BP段炎症因子表达水平的变化,探讨DJB术后ZDF大鼠BP肠袢炎症状态及炎症状态变化对糖尿病大鼠血糖稳态的改善作用及其可能机制。
1 材料与方法 1.1 动物、主要仪器和试剂20只雄性8周龄T2DM模型ZDF大鼠,购于北京维通利华实验动物技术有限公司,动物合格证号:11400700232583;购买动物后先适应环境1周,20只大鼠随机分为DJB组(n=10)和假手术组(n=10),每组均存活8只大鼠进行后续实验。免疫组织化学超敏二步法试剂盒购自北京中杉金桥公司,ELISA试剂盒购自上海酶联生物有限公司,氯胺酮为山东方明药业集团股份有限公司产品,TRIzol逆转录试剂盒、SYBR Green Real-time PCR Master Mix购自日本Toyobo公司,小鼠anti-AMPK和小鼠anti-p-AMPK单克隆抗体购自美国CST公司。血糖仪购于美国Roche公司,数显恒温水浴锅HH-2购于中国常州国华电器有限公司,实时荧光定量PCR仪为美国Roche公司产品。
1.2 动物分组和手术方式ZDF大鼠适应环境1周后,按照分组进行DJB和假手术干预。手术方式见文献[12-13]。DJB组大鼠术前禁食18 h,称体质量,氯胺酮(0.4 mL·100 g-1)腹腔注射麻醉大鼠。在胃的幽门部和十二指肠连接处剪开,封闭十二指肠残端,屈氏韧带下10 cm处切断空肠,远端的空肠上提与胃的幽门部连接,近端的空肠连接到屈氏韧带以下15 cm处。术后抗炎补液处理,缝合处每天用酒精棉球消毒,持续1周。假手术组大鼠的手术操作除了不实施十二指肠空肠旷置,其他与DJB组操作相似。
1.3 大鼠术前术后空腹血糖水平检测(fasting blood glucose,FBG)分别在术前1周和术后1~6周采用血糖仪检测2组大鼠FBG水平,记录数值。
1.4 ELISA法检测大鼠术后空腹血清胰岛素(FINS)水平术后6周大鼠內眦静脉取血,将血放入抗凝管,室温静置4 h后,血液样本以3 500 r·min-1、4℃离心,取上清液,严格按照ELISA试剂盒说明书操作。根据空腹血糖及FINS水平,计算术后大鼠胰岛素抵抗指数(HOMA-IR)及胰岛素敏感指数(HOMA-ISI)。HOMA-IR=(FINS×FBG)/22.5,HOMA-ISI=1/ (GLU×FINS)。
1.5 HE染色观察大鼠术后BP肠袢中炎性细胞形态表现术后6周,取DJB组大鼠BP肠袢组织及假手术组相应组织置于4%多聚甲醛中固定,常规石蜡包埋,莱卡石蜡切片机切片(3.5 μm)。65 ℃烤片;二甲苯中脱蜡20 min×3次,用吸水纸吸干液体;100%乙醇5 min×3次,用吸水纸吸干液体;95%乙醇5 min×3次,用吸水纸吸干液体;流动的自来水中2 min,用吸水纸吸干水分;苏木素染色5 min;流动的自来水中稍洗;1%盐酸酒精溶液分化约5 s(切片由蓝变红);自来水冲洗返蓝约30 min;0.5%伊红染色2 min,75%乙醇脱水5 min×1次,用吸水纸吸干液体;85%乙醇脱水5 min×1次,用吸水纸吸干液体;95%乙醇脱水5 min×1次,用吸水纸吸干液体;100%乙醇脱水3 min×2次,用吸水纸吸干液体;二甲苯中透明5 min×3次,用吸水纸吸干多余液体;中性树胶封片。Olympus显微镜光镜下分析大鼠BP肠袢炎性细胞形态表现。
1.6 免疫组织化学法检测大鼠BP肠袢中AMPK和pAMPK mRNA表达水平手术后6周处死大鼠,4%多聚甲醛固定2组大鼠BP肠袢组织,石蜡包埋,切片(3.5 μm),石蜡切片置于63℃烘箱中,烘片2 h,脱蜡至水。用pH 7.4的PBS冲洗3次,每次3 min。取一定量柠檬酸盐缓冲液(pH=6.0),加入染色盒中,将脱蜡水化后的组织切片置于染色盒内耐高温塑料切片架上,放入缓冲液高温高压处理10 min,取出染色盒室温自然冷却,从缓冲液中取出玻片,先用蒸馏水冲洗2次,之后用PBS冲洗3×3 min。滴加3% H2O2,室温下孵育10 min,PBS冲洗3 min×3次。除去PBS液,滴加血清封闭液,室温孵育5 min。PBS冲洗3 min×3次,除去PBS液,每张切片加入100 μL稀释后的一抗AMPK(1:100)和pAMPK(1:100),孵育过夜。PBS冲洗3 min×5次。除去PBS液,每张切片加100 μL一抗放大剂,室温下孵育20 min。PBS冲洗3 min×3次,除去PBS液,每张切片加入100 μL酶标二抗,室温下孵育10 min。PBS冲洗3 min×5次除去PBS液,每张切片加入50 μL新鲜配制的DAB显色液,显微镜下观察3~5 min。苏木素复染,1%HCl酒精分化,自来水冲洗,蓝化,切片经梯度酒精(75%→85%→95%→100%)脱水干燥,二甲苯透明,中性树胶封固,晾干后Olympus显微镜观察拍照。
1.7 实时荧光定量PCR(QRT-PCR)法检测炎性相关因子表达水平术后6周,留取DJB大鼠BP肠袢组织和假手术组大鼠相应组织,立即放入液氮冻存。Trizol提取总RNA,定量后取1 μg逆转录为cDNA、炎症相关因子白细胞介素1β(IL-1β)、白细胞介素6(IL-6)、肿瘤坏死因子α(TNF-α)、核因子κB(NF-κB)、白细胞介素10(IL-10)以及磷酸腺苷(AMP)激活的蛋白激酶(AMPK)引物序列见表 1。PCR反应条件:预变性95℃、10 min;变性95℃、10 s,退火60℃、30 s,共40个循环。熔解曲线:从55 ℃到95 ℃,每隔10 s增加0.5℃。采用LightCycler 480Ⅱ(Roche)实时定量PCR仪进行检测,以GAPDH作为内参基因。
| Gene | Forward primer (5'-3') | Reverse primer (5'-3') |
| IL-1β | CTCACAAGCAGAGCACAAGC | CTCACAAGCAGAGCACAAGC |
| IL-6 | TCTGCAAGAGACTTCCATCCA | AGTCTCCTCTCCGGACTTGT |
| TNF-α | GGTGCCTATGTCTCAGCCTC | GGTGCCTATGTCTCAGCCTC |
| IL-10 | TGCACTACCAAAGCCACAAG | TGATCCTCATGCCAGTCAGT |
| NF-kp65 | ACCTGGCATCTGTGGACAAC | TCTCCTGAGAGACCATTGGGA |
| AMPK | TGTAGAGCAATCAAGCAGTTGGA | TCCTTCGTACACGCAAATAATAGG |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
采用Graph Pad Prism 5.0 Software统计软件进行统计学分析。2组大鼠FBG水平,空腹胰岛素水平,HOMA-IR、HOMA-ISI、AMPK和pAMPK mRNA表达水平以及炎性相关因子IL-1β、IL-6、TNF-α、NF-κB和IL-10 mRNA表达水平以x±s表示,组间比较采用独立样本t检验。以ɑ=0.05为检验水准。
2 结果 2.1 术后2组大鼠FBG水平与假手术组比较,DJB组术后2周大鼠FBG水平降低(t=3.205, P<0.05)。与术前比较,DJB组大鼠FBG水平手术后第2周明显降低(t=3.798, P<0.05),而假手术组大鼠手术前后FBG水平均无明显变化。见表 2。
| [n=8, x±s, cB/nmol·L-1] | |||||
| Group | FBG level | ||||
| Before operation | 1 week after operation | 2 weeks after operation | 4 weeks after operation | 6 weeks after operation | |
| Shamoperation | 7.98±0.32 | 7.25±0.37 | 7.73±0.24 | 4.62±0.46 | 7.55±0.38 |
| DJB | 8.03±0.47 | 7.29±0.25 | 6.58±0.85*△ | 5.28±0.94*△ | 5.52±0.58*△ |
| *P < 0.05 compared with sham operation; △P < 0.05 compared with before operation in the same group。 | |||||
2组大鼠术前(9.718 mU·L-1±1.177 mU·L-1 vs 9.716 mU·L-1 ±0.346 mU·L-1;t=0.601,P>0.05)和术后6周(9.72 mU·L-1± 0.58 mU·L-1 vs 9.38 mU·L-1±1.18 mU·L-1;t=0.641,P>0.05)血清胰岛素水平比较差异均无统计学意义。与假手术组比较,DJB组大鼠HOMA-IR明显降低(2.38±0.002 vs 3.26±0.001;t=4.441,P<0.05); DJB组大鼠HOMA-ISI明显高于假手术组(0.03±0.005 vs 0.01±0.004;t=-8.65,P<0.05)。
2.3 2组大鼠BP肠袢中炎性细胞形态表现DJB组大鼠肠壁炎性细胞浸润明显减轻,而假手术组大鼠炎性细胞浸润多于DJB组。见图 1(插页五)。
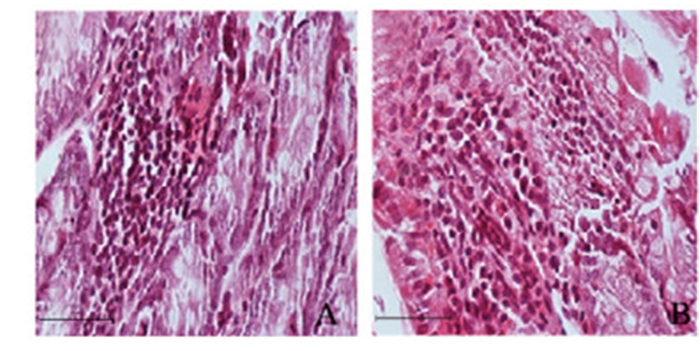
|
| 图 1 术后6周假于术组(A)和DJB组(B)大鼠BP肠袢中炎性细胞形态表现(Bar=50 μ/m) Figure 1 Morphology of inflammatory cells of rats in sham operation group (A) and DJB group (R) 6 weeks after operation (Bar =50 μm) |
|
|
DJB手术组大鼠BP肠袢中AMPK mRNA水平高于假手术组(0.20±0.02 vs 0.13±0.10;t=-8.874,P<0.05)。DJB组大鼠BP段肠袢中pAMPK表达水平明显高于假手术组(0.18±0.03 vs 0.11±0.05;t=-8.782,P<0.05)。见图 2(插页五)。
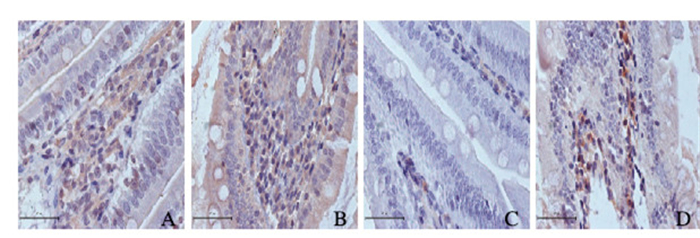
|
| A, B: AMPK; C, D: pAMPK; A, C: Sham operation group; B, D: DJB group. 图 2 免疫组织化学法检测2组大鼠BP肠袢中AMPK和pAMPK mRNA的表达(Bar=50 μm) Figure 2 Expressions of AMPK and pAMPK mRNA in BP lamb of rats in sham operation group and DJB group 6 weeks after operation (Bar=50 μm) |
|
|
与假手术组比较,DJB组大鼠促炎性因子包括IL-1β表达水平降低(t=35.47,P<0.05),IL-6表达水平降低(t=8.997,P<0.05),TNF-ɑ表达水平降低(t=21.751,P<0.05),NF-κB表达水平降低(t=5.390,P<0.05),IL-10表达水平升高(t= -12.000,P<0.05)。见表 3。
| (n=8, x±s) | |||||||||||||||
| Group | IL-lβ | IL-6 | TNF-α | NF-κB | IL-10 | ||||||||||
| Before operation | 6 weeks after operation | Before operation | 6 weeks after operation | Before operation | 6 weeks after operation | Before operation | 6 weeks after operation | Before operation | 6 weeks after operation | ||||||
| Sham operation | 0.80±0.22 | 0.80±0.21 | 0.72±0.05 | 0.72±0.10 | 0.52±0.02 | 0.05±0.02 | 0 52±0.04 | 0.50±0.21 | 0.30±0.01 | 0.31±0.03 | |||||
| DJB | 0.80±0.13 | 0.34±0.02*△ | 0.72±0.03 | 0.55±0.02*△ | 0.05±0.02 | 0.32±0.01*△ | 0.52±0.12 | 0.39±0.04 *△ | 0.32±0.10 | 0.42±0.02*△ | |||||
| *P < 0.05 compared with sham operation; △P < 0.05 compared with before operation in the same group. | |||||||||||||||
T2DM是以胰岛素抵抗和胰岛β细胞受损为主要病理特征,且与肥胖、高脂血症反应等密切相关。研究[14]显示:DJB能够有效降低T2DM大鼠FBG水平而不影响胰岛素分泌,这与改善胰岛素DJB经西方特点饮食(高糖和高脂)诱导的肥胖大鼠干预后,胰岛素分泌水平升高。本研究结果显示:DJB干预ZDF大鼠能够降低胰岛素抵抗,提高胰岛素敏感性,而不影响胰岛素分泌。T2DM常常伴有血液中急性期反应标志物的浓度升高,但不同于损伤、感染等应激因素作用下的炎症反应,T2DM是机体的先天性免疫反应(innate immune system,IIS)激活和慢性炎症的过程,该过程在糖尿病的发病机制中起媒介作用。代谢病的炎症研究是近年来的研究热点,Donath等[16]提出T2DM是炎症性疾病,炎症因子升高导致胰岛素抵抗的发生。研究[17]显示:DJB可干预T2DM降低体内炎性因子水平,从而改善血糖和相关的心血管并发症。研究[18]报道:T2DM患者应用IL-1受体拮抗剂,使得炎性因子如IL-6、C反应蛋白水平明显降低,开创了使用IL-1中和方法治疗T2DM先例。Teng等[14]通过使用IL-1受体拮抗剂治疗1型糖尿病发现效果甚微,但是对T2DM的作用却较为明显,IL-1可以降低糖化血红蛋白、CRP和细胞因子,并升高C肽水平。本课题组以ZDF大鼠作研究对象和建立DJB干预模型发现:ZDF大鼠BP肠袢有大量炎性细胞,DJB干预后,HE染色结果显示:大鼠BP肠袢部位炎性细胞数降低,炎性因子IL-1β、IL-6、TNF-α和NF-κB表达水平降低,而IL-10表达水平明显升高。AMPK参与重要器官葡萄糖代谢调节(肝脏和肠等),AMPK信号通路通过NF-κB和JAK-STAT等途径参与炎症调节,并且与胰岛素信号通路存在联系。研究[19-21]表明:AMPK过表达或AMPK激活剂均具有胰岛素增敏剂效应。本研究结果显示:ZDF大鼠通过DJB干预后,BP肠袢AMPK信号分子表达水平增多,可能BP肠袢AMPK信号途径如炎性信号途径参与了ZDF大鼠胰岛素抵抗的改善作用。DJB降低ZDF大鼠BP肠袢炎性细胞数和炎性因子表达水平,提高抗炎性因子表达水平,同时使AMPK信号分子表达水平升高,AMPK信号途径可能参与了该部位炎性状态改善。本课题组发现:DJB不影响ZDF大鼠胰岛素的分泌,而是通过改善胰岛素抵抗和提高胰岛素敏感性改善T2DM。本研究结果提示:BP肠袢靶部位的炎症抑制剂药物研发以及AMPK增强剂药物研发均有可能有益于T2DM的治疗,为糖尿病的精准治疗提供了有利借鉴。
| [1] | 中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2013年版)[J]. 中国糖尿病杂志, 2014, 22(8): 2–42. |
| [2] | Cummings DE. Metabolic surgery for type 2 diabetes[J]. Nat Med, 2012, 18(5): 656–658. DOI:10.1038/nm.2773 |
| [3] | Rubino F, Nathan DM, Eckel RH, et al. Metabolic Surgery in the Treatment algorithm for type 2 diabetes[J]. Diabetes Care, 2016, 39(11): 861–877. |
| [4] | Patriti A, Facchiano E, Donini A. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes:a new perspective for an old disease[J]. Ann Surg, 2004, 240(2): 1–11. |
| [5] | Zand H, Morshedzadeh N, Naghashian F. Signaling pathways linking inflammation to insulin resistance[J]. Diabetes Metab Syndr, 2017(16): 30297. |
| [6] | Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis[J]. Science, 2013, 339(6116): 172–177. DOI:10.1126/science.1230721 |
| [7] | Khodabandeloo H, Gorganifiruzjaee S, Panahi G, et al. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction[J]. Transl Res, 2016, 167: 228. DOI:10.1016/j.trsl.2015.08.011 |
| [8] | Chen L, Chen R, Wang H, et al. Mechanisms linking inflammation to insulin r esistance[J]. Int J Endocrinol, 2015, 2015: 508409. |
| [9] | Aggarwal R, Harling L, Efthimiou E, et al. The effects of bariatric surgery on cardiac structure and function:a systematic review of cardiac imaging outcomes[J]. Obes Surg, 2016, 26(5): 1030–1040. DOI:10.1007/s11695-015-1866-5 |
| [10] | 胡春晓. 炎症在十二指肠空肠旁路术缓解2型糖尿病中的作用及机制研究[D]. 济南: 山东大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10422-1014310327.htm |
| [11] | Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat[J]. Methods Mol Biol, 2012, 933: 103–123. |
| [12] | 潘瑞艳, 严庆涛, 曲俊生, 等. 大网膜瓣包绕胃肠道吻合口防治糖尿病大鼠消化系吻合口漏的疗效[J]. 世界华人消化杂志, 2013, 21(29): 3107–3111. |
| [13] | 匡少金, 季万胜, 柳蕾, 等. 十二指肠空肠旁路术对2型糖尿病大鼠胰岛β细胞功能恢复的作用[J]. 实用医学杂志, 2015, 31(2): 178–181. |
| [14] | Liu T, Zhong MW, Liu Y, et al. Diabetes recurrence after metabolic surgeries correlates with re-impaired insulin sensitivity rather than beta-cell function[J]. World J Gastroenterol, 2017, 23(19): 3468–3479. DOI:10.3748/wjg.v23.i19.3468 |
| [15] | Araujo AC, Bonfleur ML, Balbo SL, et al. Duodenal-jejunal bypass surgery enhances glucose tolerance and beta-cell function in western diet obese rats[J]. Obes Surg, 2012, 22(5): 819–826. DOI:10.1007/s11695-012-0630-3 |
| [16] | Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease[J]. Nat Rev Immunol, 2011, 11(2): 98–107. DOI:10.1038/nri2925 |
| [17] | Chen X, Huang Z, Ran W, et al. Type 2 diabetes mellitus control and atherosclerosis prevention in a non-obese rat model using duodenal-jejunal bypass[J]. Exp Ther Med, 2014, 8(3): 856–862. |
| [18] | Malozowski S, Sahlroot JT. Interleukin-1-receptor antagonist in type 2 diabetes mellitus[J]. N Engl J Med, 2007, 357(3): 302–303. DOI:10.1056/NEJMc071324 |
| [19] | Fillmore N, Jacobs DL, Mills DB, et al. Chronic AMP-activated protein kinase activation and a high-fat diet have an additive effect on mitochondria in rat skeletal muscle[J]. J Appl Physiol, 2010, 109(2): 511. DOI:10.1152/japplphysiol.00126.2010 |
| [20] | Liong S, Lappas M. Activation of AMPK improves inflammation and insulin resistance in adipose tissue and skeletal muscle from pregnant women[J]. J Physiol Biochem, 2015, 71(4): 703. DOI:10.1007/s13105-015-0435-7 |
| [21] | 颜英, 汤绍辉, 黄秋燕, 等. 二甲双胍与2型糖尿病患者大肠癌发病风险关系的Meta分析[J]. 解放军医学杂志, 2015, 40(7): 582–586. DOI:10.11855/j.issn.0577-7402.2015.07.14 |
 2017, Vol. 43
2017, Vol. 43


