扩展功能
文章信息
- 李彩, 魏洁, 甄永占, 代明鹤, 胡刚, 郭立新, 林雅军
- LI Cai, WEI Jie, ZHEN Yongzhan, DAI Minghe, HU Gang, GUO Lixin, LIN Yajun
- 赖氨大黄酸对KK/HlJ糖尿病小鼠胰岛素抵抗的改善作用及其机制
- Improvement effect of rhein lysinate on insulin resistance of KK/HlJ diabetic mice and its mechanism
- 吉林大学学报(医学版), 2017, 43(06): 1074-1079
- Journal of Jilin University (Medicine Edition), 2017, 43(06): 1074-1079
- 10.13481/j.1671-587x.20170602
-
文章历史
- 收稿日期: 2017-03-07
2. 北京医院国家老年医学中心 卫生部老年医学重点实验室, 北京 100730;
3. 华北理工大学基础医学院组织学与胚胎学教研室, 河北 唐山 063000
2. National Center of Gerontology, Beijing Hospital, Key Laboratory of Geriatrics, Ministry of Health, Beijing 100730, China;
3. Department of Histology and Embryology, College of Basic Medical Sciences, North China University of Science and Technology, Tangshan 063000, China
糖尿病(diabetes mellitus,DM)是一种慢性代谢紊乱疾病,分为1型和2型。2型DM约占DM病例的95%, DM具有胰岛素抵抗。胰岛素抵抗由遗传和环境因素引起,机体对胰岛素生理作用的反应性降低。KK/HlJ小鼠在DM饮食喂养条件下容易出现血糖水平升高,并发胰岛素抵抗[1-2],因此本研究选择KK/HlJ DM小鼠为研究对象。赖氨大黄酸(rhein lysinate,RHL)是本室合成的由大黄酸与赖氨酸反应生成的盐。本品较大黄酸更易溶于水,溶解度为8 g·L-1[3]。以往研究[4-6]表明:RHL能够通过抑制炎症反应对早衰小鼠肾脏和DM小鼠肝脏起保护作用,但其对胰腺组织及胰岛素抵抗的治疗作用尚不明确。与本课题组先前研究比较,本研究首次在KK/HlJDM小鼠模型中探讨了RHL对胰腺组织的保护作用和对胰岛素抵抗的改善作用,旨在为RHL治疗DM提供理论依据。
1 材料与方法 1.1 实验动物和主要试剂雄性C57BL/J小鼠12只;雄性KK/HlJ小鼠36只,体质量(20±2) g,6~8周龄。动物合格证号:SCXK(京)2014-0004。DM专属饲料(水分≤8.0%、粗蛋白≥18.0%、粗脂肪≥6.0%、粗纤维≤5.0%、粗灰分≤7.0%以及钙、磷等微量元素),链脲佐菌素(streptozotocin,STZ,美国Sigma公司),小鼠C反应蛋白(C reactive protein,CRP)、小鼠肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)和小鼠白细胞介素6(interleukin-6,IL-6)酶联免疫吸附测定(enzyme linked immunosorbent assay,ELISA)检测试剂盒(武汉华美生物工程有限公司),胰岛素、磷脂酰肌醇3激酶(phosphatidylinositol 3-kinase,PI3K)、p-PI3K(p85)、蛋白激酶B(protein kinase B,AKT)、p -AKT(Ser473)、糖原合成酶激酶-3β(glycogen synthase kinase-3β,GSK-3β)、p-GSK-3β(Ser9)、β-actin抗体、辣根过氧化物酶(horseradish peroxidase,HRP)标记的山羊抗兔或山羊抗小鼠IgG抗体(美国Cell Signaling Technology公司),预染蛋白Marker(美国Fermentas公司),即用型SABC-AP(兔IgG)免疫组织化学试剂盒(武汉博士德生物工程有限公司)。
1.2 动物分组和给药方式12只C57BL/J小鼠作为正常对照组,将36只KK/HlJ小鼠随机分为3组,每组12只,分别为模型组、低剂量RHL治疗组(25 mg·kg-1)和高剂量RHL治疗组(50 mg·kg-1)。模型组、低和高剂量RHL治疗组小鼠在接受治疗前,每天腹腔注射50 mg·kg-1STZ,连续注射5 d;然后分别灌胃给予生理盐水、25和50 mg·kg-1RHL,同时喂以DM专属饲料。正常对照组小鼠也灌胃给予生理盐水,持续给药16周,每周称量小鼠体质量1次。
1.3 各组小鼠生化指标测定各组小鼠末次给药前禁食12 h,给药1 h后摘眼球取血,离心取血清,采用日立全自动生化分析仪检测空腹血糖(FBG)、总胆固醇(TC)和甘油三酯(TG)水平。采用ELISA试剂盒分别检测各组小鼠血清中胰岛素、CRP及肝脏组织中TNF-α和IL-6水平。
1.4 各组小鼠胰腺组织形态表现观察16周后处死小鼠,取小鼠部分胰腺组织立即置入新配置的4%多聚甲醛溶液中固定48 h,制备石蜡切片,进行常规HE和免疫组织化学染色。免疫组织化学染色步骤参照试剂盒说明书进行。胰腺组织中胰岛的棕色信号表示胰岛素染色阳性,根据棕色信号的强弱和多少间接判断胰岛素水平。胰腺组织中棕色颗粒状物质为不溶的胰岛。随机选取5个200倍镜视野,采用病例图像分析系统计数胰岛个数及胰岛体积,在显微镜下观察胰岛免疫组织化学染色信号强弱。
1.5 蛋白质提取和Western blotting检测16周后处死小鼠,取小鼠部分肝脏组织,加入RIPA组织裂解液和蛋白酶抑制剂cocktail,使用IKA高速组织匀浆机匀浆。12 000 r·min-1离心20 min,收集上清于新的微量离心管中,采用BCA法测定蛋白浓度。各取样品等量总蛋白40μg在10%十二烷基磺酸钠-聚丙烯酰胺凝胶(SDS-PAGE)上进行电泳。电泳后转移到硝酸纤维素膜上,5%牛血清白蛋白封闭1 h,一抗4℃孵育过夜,洗涤5次,加入二抗孵育2 h,洗涤5次,在膜上滴加化学发光剂,采用凝胶成像仪照相。采用Scion image软件对Western blotting条带蛋白的灰度值进行分析计算。
1.6 统计学分析采用SPSS 13.0统计软件对数据进行统计学分析。所有计量资料均符合正态分布。各组小鼠体质量、FBG、胰岛素、CRP、TNF-α和IL-6水平以x ± s表示,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠体质量和FBG、TG及TC水平实验结束时,模型组小鼠体质量较正常对照组升高(P < 0.05);高剂量RHL治疗组小鼠体质量较模型组降低(P < 0.05),但与正常对照组比较差异无统计学意义(P>0.05)。模型组小鼠FBG水平较正常对照组升高(P < 0.05);低和高剂量RHL治疗组小鼠FBG水平较模型组均降低(P < 0.05),但仍高于正常对照组(P < 0.05)。模型组小鼠TG和TC水平较正常对照组明显升高(P < 0.05);低和高剂量RHL治疗组小鼠TG和高剂量RHL治疗组小鼠TC水平均低于模型组(P < 0.05),但仍高于正常对照组(P < 0.05)。低和高剂量RHL治疗组小鼠体质量和FBG、TG及TC水平比较差异无统计学意义(P>0.05)。见表 1。
| (n=12, x ±s) | ||||
| Group | Body weight (m/g) | FBG [cB/(mmol·L-1)] | TG [cB/(mmol·L-1)] | TC [cB/(mmol·L-1)] |
| Normal control | 47.0±3.4 | 6.53±0.51 | 0.89±0.08 | 1.82±0.31 |
| Model | 49.5±1.3* | 12.36±3.00* | 2.78±0.21* | 7.57±0.54* |
| Low dose of RHL | 49.0±1.4* | 9.53±2.81*△ | 1.87±0.12*△ | 6.01±0.48* |
| High dose of RHL | 46.0±3.7△ | 8.50±1.90*△ | 1.55±0.13*△ | 5.58±0.42*△ |
| *P < 0.05 vs normal control group; △P < 0.05 vs model group. | ||||
模型组小鼠胰岛素水平较正常对照组升高(P < 0.01),低和高剂量RHL治疗组小鼠胰岛素水平与模型组比较差异无统计学意义(P>0.05)。模型组小鼠血清CRP水平较正常对照组升高(P < 0.01);低和高剂量RHL治疗组小鼠血清CRP水平较模型组均降低(P < 0.01),但仍高于正常对照组(P < 0.01)。模型组小鼠肝脏组织中TNF-α和IL-6水平较正常对照组升高(P < 0.01);低和高剂量RHL治疗组小鼠肝脏组织中TNF-α和IL-6水平较模型组均降低(P < 0.01),但仍高于正常对照组(P < 0.01)。与低剂量RHL治疗组比较,高剂量RHL治疗组小鼠血清CRP水平及肝脏组织中TNF-α和IL-6水平降低(P < 0.01)。见表 2。
| (n=12, x ±s) | ||||
| Group | Insulin [λB/(μIU·L-1)] | CRP [ρB/(mg·L-1)] | TNF-α[ρB/(mg·L-1)] | IL-6 [ρB/(mg·L-1)] |
| Normal control | 15.2±1.1 | 0.23±0.02 | 64±5 | 3.8±0.3 |
| Model | 78.1±5.3* | 15.20±1.20* | 263±21* | 23.4±1.9* |
| Low dose of RHL | 76.6±7.1* | 10.50±0.80*△ | 187±17*△ | 16.8±1.5*△ |
| High dose of RHL | 75.2±5.8* | 8.10±0.70*△# | 124±11*△# | 9.7±0.8*△# |
| *P < 0.01 vs normal control group; △P < 0.01 vs model group; #P < 0.01 vs low dose of RHL group. | ||||
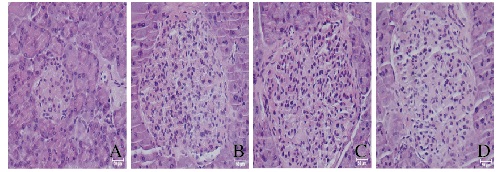
HE染色结果显示:正常对照组小鼠胰岛体积很小,数量很少(平均0.4个胰岛/200倍视野),而模型组小鼠胰岛体积很大,数量很多(平均1.4个胰岛/200倍视野)。模型组小鼠胰岛面积为(0.23±0.02)mm2, 数量为(1.4±0.3)个胰岛/200倍视野;低剂量RHL治疗组小鼠胰岛面积为(0.20±0.02)mm2,数量为(1.5±0.3)个胰岛/200倍视野; 高剂量RHL治疗组小鼠胰岛面积为(0.19±0.02)mm2,数量为(1.4±0.2)个胰岛/200倍视野。低和高剂量RHL治疗组小鼠胰岛体积和数量与模型组比较差异无统计学意义(P>0.05)。正常对照组小鼠胰岛结构完整,轮廓清晰可见;模型组小鼠胰岛结构紊乱,出现炎症浸润;低和高剂量RHL治疗组小鼠胰岛形态有一定恢复,偶见炎症浸润,但与模型组差异不大。见图 1(插页一)。
|
| A: Normal control group; B: Model group; C: Low dose of RHL group; D: High dose of RHL group. 图 1 各组小鼠胰岛组织病理形态表现(HE, ×200) Figure 1 Pathomorphology of islet tissue of mice in various groups(HE, ×200) |
|
|
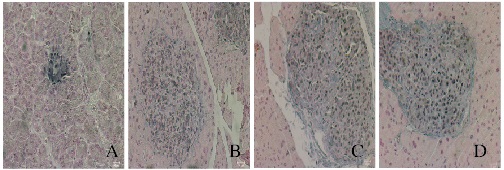
胰岛素免疫组织化学染色结果显示:正常对照组小鼠胰岛染色较深,模型组与低和高剂量RHL治疗组染色均较淡。模型组小鼠胰岛内可见大量褐色颗粒状物质,低剂量RHL治疗组小鼠棕色颗粒状物质明显减少,而正常对照组小鼠和高剂量RHL治疗组小鼠胰岛组织中未见棕色颗粒状物质。见图 2(插页一)。
|
| A: Normal control group; B: Model group; C: Low dose of RHL group; D: High dose of RHL group. 图 2 各组小鼠胰岛中胰岛素蛋白的表达(免疫组织化学, ×200) Figure 2 Expressions of insulin protein in islet of mice in various groups(Immunohistochemistry, ×200) |
|
|
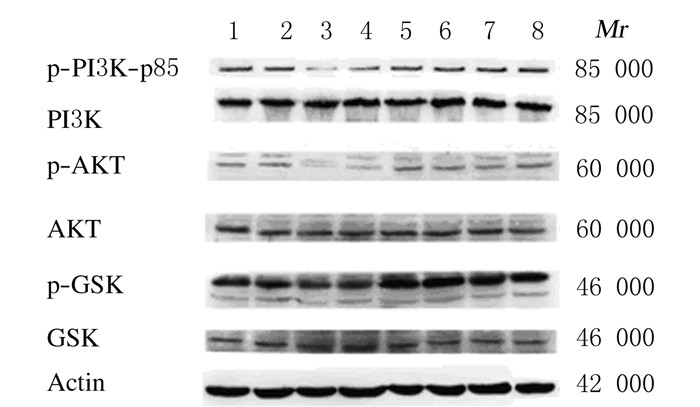
模型组小鼠肝脏组织中PI3K、AKT和GSK-3β磷酸化水平较正常对照组降低(P < 0.05),低和高剂量RHL治疗组小鼠肝脏组织中PI3K、AKT和GSK-3β磷酸化水平较模型组明显升高(P < 0.05)。低和高剂量RHL组肝脏组织中PI3K、AKT和GSK-3β磷酸化水平比较差异无统计学意义(P>0.05)。见图 3和表 3。
|
| Lane 1-2: Normal control group; Lane 3-4: Model group; Lane 5-6: Low dose of RHL group; Lane 7-8: High dose of RHL group. 图 3 Western blotting法检测各组小鼠肝脏组织中糖原合成相关蛋白表达的电泳图 Figure 3 Electrophoregram of expressions of glycogen synthesis related proteinsin liver tissue of mice in various groups |
|
|
| (n=12, x ±s) | |||
| Group | p-PI3K/PI3K | p-AKT/AKT | p-GSK/GSK |
| Normal control | 100.0±8.7 | 100.0±11.3 | 100.0±12.6 |
| Model | 31.3±2.8* | 22.6±2.7* | 45.1±3.9* |
| Low dose of RHL | 88.4±9.3△ | 97.5±12.1△ | 89.6±10.8△ |
| High dose of RHL | 105.3±13.9△ | 108.6±14.7△ | 102.9±15.2△ |
| *P < 0.05 vs normal control group; △P < 0.05 vs model group. | |||
2型DM是常见的全身性内分泌/代谢性疾病,其主要病因是胰岛素分泌相对或绝对不足以及外周组织胰岛素抵抗[7]。KK/HlJ小鼠是DM易感小鼠且伴随严重胰岛素抵抗[8-9],因此本研究以KK/HlJ小鼠作为研究对象,为了能够快速成模,本研究同时给予KK/HlJ小鼠腹腔注射低剂量STZ。与C57BL/J正常对照小鼠比较,KK/HlJ DM小鼠FBG和胰岛素水平明显升高,胰岛素水平升高更为明显,是C57BL/J正常对照小鼠的5倍,表明KK/HlJ DM小鼠存在很严重的胰岛素抵抗。为了进一步探索胰岛素抵抗的发生机制,本研究首先观察RHL对KK/HlJ DM小鼠胰岛的保护作用,HE染色发现:与正常对照组比较,模型组小鼠胰岛体积很大,数量很多,且胰岛结构紊乱,出现炎症浸润;给予低和高剂量RHL治疗后仅炎症略有改善,其他方面无改变。免疫组织化学染色结果显示:正常对照小鼠胰岛中胰岛素的表达水平较高,而模型组小鼠胰岛素的表达水平明显降低,且胰岛中呈现大量棕褐色颗粒状物质;当给予低和高剂量RHL治疗后,胰岛素水平并无明显改善,但低剂量RHL能够使胰岛中棕褐色颗粒状物质明显减少,高剂量RHL能够使胰岛中棕褐色颗粒状物质消失,这是否提示RHL对胰岛有保护作用尚待进一步研究。
本研究结果显示:低和高剂量RHL在对胰岛素无影响的情况下,能够明显降低血糖水平,说明RHL可能改善了外周组织的胰岛素抵抗。研究[10]表明:外周胰岛素抵抗主要发生在肝脏、脂肪组织和骨骼肌,因为这些组织含有丰富的胰岛素受体,其可以结合胰岛素从而发挥胰岛素信号通路的一系列生物学效应。研究[11]表明:机体长期处于炎症状态下能够诱发胰岛素抵抗。本研究结果显示:KK/HlJ DM小鼠CRP水平较高,表明KK/HlJ DM小鼠处于一个炎症状态。同时本课题组对小鼠肝脏组织中TNF-α和IL-6检测结果显示:KK/HlJ DM小鼠肝脏也处于较高炎症状态,与本课题组前期实验[12]结果相符,即与正常对照组比较,KK/HlJ DM小鼠肝脏脂肪变性和肝脏炎症非常明显。应用RHL治疗后,小鼠CRP、TNF-α和IL-6水平明显降低,表明RHL能够改善机体炎症状态。肝脏炎症状态是否又诱发了机体胰岛素抵抗,从而引起KK/HlJ小鼠出现高血糖、高胰岛素、高胆固醇和高甘油三酯血症?为了验证猜测,本课题组采用Western blotting法检测了糖原合成基因相关的信号通路。糖原合成主要是通过胰岛素介导的PI3K/AKT/GSK-3β信号通路完成的。当胰岛素与胰岛素受体(IR)结合后,可引起胰岛素受体底物2(IRS-2)酪氨酸的磷酸化,并进一步激活下游的PI3K、AKT和GSK-3β,促进糖原的合成[13-15]。本研究结果显示:模型组小鼠肝脏组织中PI3K、AKT和GSK-3β磷酸化水平明显降低,应用RHL治疗后能阻断其磷酸化水平降低,证明RHL能够通过激活PI3K/AKT/GSK-3β信号通路缓解KK/HlJDM小鼠肝脏胰岛素抵抗状态。这充分解释了RHL能够降低FBG、TC和TG而对胰岛素水平无影响的原因。
综上所述,RHL对KK/HlJ DM小鼠胰岛组织的保护作用尚待进一步研究,其降低FBG、TC和TG的作用主要是通过其对肝脏胰岛素抵抗的改善作用实现的。RHL有望成为治疗肝脏胰岛素抵抗的新药。
| [1] | Berndt A, Sundberg BA, Silva KA, et al. Phenotypic characterization of the KK/HlJ inbred mouse strain[J]. Vet Pathol, 2014, 51(4): 846–857. DOI:10.1177/0300985813501335 |
| [2] | Chang GR, Wu YY, Chiu YS, et al. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice[J]. Basic Clin Pharmacol Toxicol, 2009, 105(3): 188–198. DOI:10.1111/pto.2009.105.issue-3 |
| [3] | Lin YJ, Zhen YS. Rhein lysinate suppresses the growth of breast cancer cells and potentiates the inhibitory effect of Taxol in athymic mice[J]. Anticancer Drugs, 2009, 20(1): 65–72. DOI:10.1097/CAD.0b013e3283182913 |
| [4] | Hu G, Liu J, Zhen YZ, et al. Rhein lysinate increases the median survival time of SAMP10 mice:protective role in the kidney[J]. Acta Pharmacol Sin, 2013, 34(4): 515–521. DOI:10.1038/aps.2012.177 |
| [5] | Wei J, Zhen YZ, Cui J, et al. Rhein lysinate decreases inflammation and adipose infiltration in KK/HlJ diabetic mice with non-alcoholic fatty liver disease[J]. Arch Pharm Res, 2016, 39(7): 960–969. DOI:10.1007/s12272-016-0770-4 |
| [6] | Zhen YZ, Lin YJ, Li KJ, et al. Effects of rhein lysinate on D-galactose-induced aging mice[J]. Exp Ther Med, 2016, 11(1): 303–308. DOI:10.3892/etm.2015.2858 |
| [7] | 周士胜, 伦永志, 李胜范. 2型糖尿病病因可能在肝脏[J]. 大连大学学报, 2008, 29(3): 89–91. |
| [8] | Iizuka Y, Kim H, Izawa T, et al. Protective effects of fish oil and pioglitazone on pancreatic tissue in obese KK mice with type 2 diabetes[J]. Prostaglandins Leukot Essent Fatty Acids, 2016, 115: 53–59. DOI:10.1016/j.plefa.2016.10.007 |
| [9] | Muroyama K, Murosaki S, Yamamoto Y, et al. Anti-obesity effects of a mixture of thiamin, arginine, caffeine, and citric acid in non-insulin dependent diabetic KK mice[J]. J Nutr Sci Vitaminol, 2003, 49(1): 56–63. DOI:10.3177/jnsv.49.56 |
| [10] | 高啸, 彭旖旎, 朱琳, 等. 硫化氢对2型糖尿病大鼠肝脏胰岛素抵抗的影响[J]. 华中科技大学学报:医学版, 2016, 45(5): 490–495. |
| [11] | Wen L, Duffy A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes[J]. J Nutr, 2017, 147(7): 1468S–1475S. DOI:10.3945/jn.116.240754 |
| [12] | Wei J, Zhen YZ, Cui J, et al. Rhein lysinate decreases inflammation and adipose infiltration in KK/HlJ diabetic mice with non-alcoholic fatty liver disease[J]. Arch Pharm Res, 2016, 39(7): 960–969. DOI:10.1007/s12272-016-0770-4 |
| [13] | Wang X, Zhao L. Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3β signaling pathway[J]. Biochem Biophys Res Commun, 2016, 473(2): 428–434. DOI:10.1016/j.bbrc.2016.03.024 |
| [14] | Zhou L, Wang L, Yang B, et al. Protective effect of pretreatment with propofol against tumor necrosis factor-alpha-induced hepatic insulin resistance[J]. Exp Ther Med, 2015, 10(1): 289–294. DOI:10.3892/etm.2015.2496 |
| [15] | Wang X, Wang M, Li H, et al. Upregulation of miR-497 induces hepatic insulin resistance in E3 rats with HFD-MetS by targeting insulin receptor[J]. Mol Cell Endocrinol, 2015, 416: 57–69. DOI:10.1016/j.mce.2015.08.021 |
 2017, Vol. 43
2017, Vol. 43


