扩展功能
文章信息
- 周浩泉, 储新民, 戴媛媛, 代传林, 潘家华
- ZHOU Haoquan, CHU Xinmin, DAI Yuanyuan, DAI Chuanlin, PAN Jiahua
- 婴幼儿非发酵菌感染的临床特征和耐药分析
- Clinical characteristics and drug resistance analysis of non-fermentative bacteria infection in infants
- 吉林大学学报(医学版), 2017, 43(05): 975-979
- Journal of Jilin University (Medicine Edition), 2017, 43(05): 975-979
- 10.13481/j.1671-587x.20170522
-
文章历史
- 收稿日期: 2016-12-29
- 网络出版时间: 2017-09-18 14:09:27
2. 安徽医科大学附属省立医院检验科, 安徽 合肥 230001
2. Deparment of Laboratory, Anhui Provincial Hospital, Anhui Medical University, Hefei 230001, China
非发酵菌感染一直是临床医师高度重视的课题,特别是近年来细菌耐药性的增加,广泛耐药菌株的出现,使人们面临无药可用的困境,对非发酵菌感染的研究更加迫切[1-2]。婴幼儿免疫力低下,如果患有基础性疾病的同时感染此类菌,则治疗起来十分困难。国内外关于成人感染非发酵菌的临床特征以及耐药性的研究较多,而针对婴幼儿这一特殊群体感染非发酵菌的研究报道较少。本研究通过探讨婴幼儿感染非发酵菌的临床特征以及该群体感染非发酵菌的耐药性和耐药基因出现的规律,为临床诊断和合理使用抗生素提供参考依据。
1 资料与方法 1.1 临床资料本组病例为2012年1月—2016年12月本院儿科住院的91例患儿。研究对象入组标准:年龄≤3岁的住院患儿,有细菌感染症状,经实验室细菌培养证实为非发酵菌感染,临床资料齐全;排除标准:临床资料不全者。新生儿35例(早产儿19例),29日龄~1岁29例,>1岁~3岁27例。其中男性60例,女性31例。新生儿患儿主要诊断:肺炎14例,早产儿19例,败血症4例,新生儿缺氧缺血性脑病4例,新生儿肺透明膜病9例,动脉导管未闭1例,支气管肺发育不良1例。非新生儿患儿主要诊断:肺炎39例,其中重症手足口病并发呼吸机相关性肺炎(VAP)3例,重症病毒性脑炎并发医院获得性肺炎1例,唇腭裂并发吸入性肺炎1例,哮喘并发肺炎2例,免疫功能低下并发肺炎4例,并发先天性心脏病9例,心肌炎并发肺炎2例,溺水并发吸入性肺炎1例,并发多脏器功能衰竭7例,麻疹并发肺炎1例;脓毒症12例,川崎病2例,化脓性脑膜炎3例。机械通气11例,深静脉置管27例,气管插管13例,放置导尿管7例,置胃管22例。平均住院天数(19.2±2.3) d,自动出院3例,死亡1例。
1.2 菌株标本来源102份标本来源于儿科各病区的3岁及以下住院患儿,明确诊断或疑似感染者,采取血液、深部痰、支气管肺泡灌洗液、引流液和分泌物等。其中血液标本19份,深部痰67份,支气管肺泡灌洗液7份,穿刺液2份,分泌物3份,引流液3份,导管1例。采集时严格按照操作技术规范进行,及时送检。
1.3 主要试剂和仪器MH琼脂和抗菌药物纸片(英国OXOID公司),Taq酶和DNA Marker 9(大连宝生物公司),引物由上海生工合成。Vitek 2 Compect全自动微生物分析系统(法国梅里埃公司),PCR扩增仪(美国PE公司),电泳仪(英国Bio-Rad公司),凝胶成像系统(英国UVI公司)。
1.4 细菌鉴定及药敏纯培养菌落按照《全国临床检验操作规程》采用Vitek 2 Compect全自动微生物分析系统进行鉴定和稀释法药敏测试,并采用纸片法回顾性分析各抗菌药物的耐药性,判断标准参照美国2016版CLSI标准进行。鲍曼不动杆菌及铜绿假单胞菌的多重耐药(multi-drug resistantce,MDR)、广泛耐药(extensively drug resistantce,XDR)和泛耐药(pandrug resistantce, PDR)分类方法参照Magiorakos标准[3]。
1.5 耐药基因检测提取细菌组DNA:挑单个菌落于0.5 mL离心管(内已预置200 μg·L-1蛋白酶K溶液)内,56℃水浴2 h,95℃水浴10 min,12 000 r·min-1离心30s,吸取上清液作为DNA模板,-20℃冰箱备用。PCR扩增:根据说明书建立PCR体系,PCR引物序列见表 1。PCR产物送上海生工进行DNA测序,测序结果与GenBank中BLAST网站(http://www.ncbi.nlm.nih.gov/blast)进行比对分析,确定基因型别。
1.6 数据分析使用WHONET 5.6软件对药敏结果进行耐药性分析。
2 结果 2.1 感染非发酵菌患儿基本情况在感染非发酵菌的患儿中,新生儿患儿35例(38.46%),其中早产儿19例,占新生儿患者的54.28%;儿童重症监护病房患儿31例(34.06%),普儿科病房患儿25例(27.47%);机械通气11例(12.09%),置管患儿43例(47.25%);有基础性疾病患儿41例(45.05%),出现脏器功能损害16例(17.58%),自动出院3例(3.29%),死亡1例(1.09%)。
2.2 非发酵菌菌株分布取自91例婴幼儿患者的121个标本共培养出非发酵菌102株,占同期全部菌株的5.71%(102/1 785),占同期革兰阴性杆菌的9.83%(102/1 038),占同期非发酵菌的59.30%(102/172)。铜绿假单胞菌42株(41.18%),其中新生儿病房19株,儿童重症监护病房16株,普儿科病房7株;鲍曼不动杆菌33株(32.35%),分布科室为新生儿病房14株,儿童重症监护病房8株,普儿科病房11株;嗜麦芽窄食单胞菌21株(20.59%),分布科室为新生儿病房9株,重症监护病房6株,普儿科病房6株;其他少见非发酵菌6株。见表 1。
| Strain | Newborn nursery(Isolate) | PICU(Isolate) | General pediatric ward(Isolate) |
| Pseudomonas aeruginosa | 19 | 16 | 7 |
| Acinetobacter baumannii | 14 | 8 | 11 |
| Stenotrophomonas maltophilia | 9 | 6 | 6 |
| Other | 2 | 3 | 1 |
| Total | 44(43.13%) | 33(32.35%) | 25(24.50%) |
| Note: Specimen were isolated from pediatric ward in hospital, and bacterial culture was identified as non-fermentative bacteria. | |||
在所有标本中,深部痰标本67例,占标本总数的65.7%;血液标本19例,占18.6%;其他种类标本相对较少。见表 2。
| Specimen | Number(n=102) | Rate(η/%) |
| Sputum | 67 | 65.7 |
| Blood | 19 | 18.6 |
| Bronchoalveolar lavage fluid | 7 | 6.9 |
| Puncture fluid | 2 | 1.9 |
| Secretion | 3 | 2.9 |
| Drainage | 3 | 2.9 |
| Catheter | 1 | 0.9 |
| Total | 102 | 100.0 |
铜绿假单胞菌中MDR、XDR分别为11.90%和7.14%,亚胺培南耐药率为40.48%;鲍曼不动杆菌中MDR、XDR和PDR分别为39.39%、18.18%和6.06%,亚胺培南耐药率为36.36%。见表 3和4。
| [n(η/%)] | ||
| Strain | Pseudomonas aeruginosa | Acinetobacter baumannii |
| MDR | 5(11.90) | 13(39.39) |
| XDR | 3(7.14) | 6(18.18) |
| PDR | - | 2(6.06) |
| Note: The classification method refer red to the Magiorakos standard[3], and this standard did not contain Stenotrophomonas maltophilia. | ||
| (η/%) | ||
| Antibiotic | Resistance rate of Pseudomonas aeruginosa | Resistance rate of Acinetobacterbaumannii |
| Amikacin | 11.90 | 27.88 |
| Ciprofloxacin | 33.33 | 30.30 |
| Levofloxacin | 33.33 | 30.30 |
| Ceftazidime | 50.00 | 42.42 |
| Cefoperazone sulbactam | 47.62 | 33.33 |
| Piperacillin-tazobactam | 33.33 | 30.30 |
| Cefepime | 35.71 | 39.39 |
| Ampicillin-salbactam | 42.86 | 39.39 |
| Imipenem | 40.48 | 36.36 |
| Note: To be consistent with table 3, the table did not include Stenotrophomonas maltophilia. | ||
对42株铜绿假单胞菌和33株鲍曼不动杆菌中β-内酰胺类耐药基因进行检测。42株铜绿假单胞菌中β-内酰胺酶基因阳性株为17株,其中有5株同时携带4种耐药基因blaPER、blaSHV、blaTEM和blaOXA-10,该5株铜绿假单胞菌对头孢类、β内酰胺酶抑制剂和碳青霉烯类均耐药(图 1A~D)。33株鲍曼不动杆菌中有12株均携带blaOXA-51和blaOXA-23基因(图 1E和F)。见表 5和6。
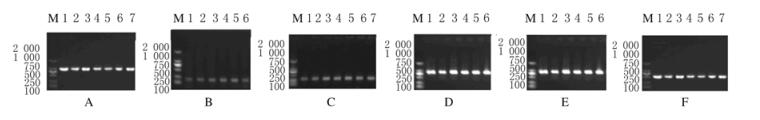
|
| A:blaPER; B.blaSHV; C:blaTEM; D:blaOXA-10; E:blaOXA-51; F:blaOXA-23, M:DL 2 000 marker 图 1 主要非发酵菌菌株耐药基因的PCR检测电泳图 Figure 1 Electrophoresis of resistance genes of main non-fermentative strains detected by PCR |
|
|
| Gene | Isolate | Positive rate(η/%) |
| blaPER | 12/42 | 28.57 |
| blaSHV | 8/42 | 19.05 |
| blaTEM | 7/42 | 16.67 |
| blaOXA-10 | 6/42 | 14.29 |
| Note: Among them, 6 strains carried blaPER, blaSHV, blaTEM and blaOXA-10, 1 strain carried blaPER, blaSHV, blaTEM, 1 strain carried blaPER, blaSHV, 4 strains carried only blaPER. | ||
| Gene | Isolate | Positive rate(η/%) |
| blaKPC | 0/33 | 0 |
| blaIMP | 0/33 | 0 |
| blaVIM | 0/33 | 0 |
| blaOXA-51 | 12/33 | 36.36 |
| blaOXA-23 | 12/33 | 36.36 |
非发酵菌常见于院内感染,儿童、老年和有基础性疾病者多发。本研究资料显示:非发酵菌感染检出率和细菌谱与国内文献报道[4-5]相似,婴幼儿感染非发酵菌菌株占同期全部菌株的5.71%(102/1 785),占同期患儿非发酵菌感染的59.30%(102/172);新生儿占本组资料的38.46%,其中54.28%患儿为早产儿;在儿童感染非发酵菌中,3岁以下患儿占比接近60%,年龄越小感染率越高,与相关文献[6]报道一致,婴幼儿感染率高与该年龄段患儿免疫功能不健全,各种屏障功能不完善有密切关系。
在本组感染非发酵菌的婴幼儿中,有基础性疾病者占比很大,接近半数,这些基础性疾病损害了患儿免疫功能,且住院时间长,易形成交叉感染。侵袭性诊治操作,特别是机械通气和各种体内置管者易发生非发酵菌感染,本组病例中置管患儿占47.25%,接近半数。侵袭性操作容易把病原菌带入体内,生长繁殖,附着于医用材料表面,并形成生物被膜,难以清除。从菌株分布看,75.38%来自新生儿病房和重症监护病房,主要原因可能与患儿年龄小、侵袭性操作多、住院时间长且多伴有基础性疾病有关。
非发酵菌感染可发生于人体各个脏器和组织,本组资料中以呼吸系统疾病为主,肺炎比例最高,其次是败血症。婴幼儿非发酵菌感染,早期无特异性临床症状或体征,易被误诊误治,即使考虑到可能是非发酵菌感染,经验性治疗往往疗效欠佳,因为非发酵菌多为耐药菌株,治疗效果差;特别是有基础性疾病或有侵袭性操作或置管患者,这些基础性疾病不能治愈,因置管而被感染的患者置管仍在体内,即使使用较为敏感的抗生素,也是无法清除细菌,临床预后不良。本组病例中死亡及放弃治疗而自动出院者共4例,占4.39%,如此差的预后在感染性疾病中是较为少见的。对于多重耐药的非发酵菌感染,不宜单药治疗,应该联合2或3种抗生素,既可提高治疗效果又避免了新耐药菌的生成[7-8]。
本组资料中MDR和XDR铜绿假单胞菌为19.04%,与国内文献[9]报道结果相似,其耐药性主要与产β-内酰胺酶有关[10]。本研究对铜绿假单胞菌的β内酰胺酶blaPER、blaSHV、blaTEM和blaOXA-10基因进行检测结果显示:42株铜绿假单胞菌中,12株碳青霉烯耐药菌均为β内酰胺酶阳性;在耐药菌中所检出的ESBLs基因主要为blaPER型(28.57%),其他如blaSHV型(19.05%)、blaTEM型(16.67%)、blaOXA-10型(14.29%)则相对较低。本研究资料中铜绿假单胞菌ESBLs阳性率高于广州地区[11],可能与铜绿假单胞菌耐药质粒所携带ESBLs的地域间差异性表达有一定关系。17株碳青霉烯耐药菌中,5株为耐药基因阴性,推测可能与不常见β内酰胺酶的产生有关,但结论尚需进一步论证。
本组资料中63.63%的鲍曼不动杆菌为MRD、XDR和PDR,β内酰胺酶耐药性与其质粒携带的编码基因有关[12],与相关文献[13-14]比较,本组资料中鲍曼不动杆菌对亚胺培南耐药率相对较低,可能与患儿来源不同有关。本文作者对鲍曼不动杆菌常见的β内酰胺酶blaKPC、blaIMP、blaVIM、blaOXA-51和blaOXA-23基因进行检测,结果显示blaKPC、blaIMP和blaVIM基因均为阴性,而12株哌拉西林-他唑巴坦、亚胺培南均耐药的鲍曼不动杆菌均为blaOXA-51和blaOXA-23阳性。OXA-23基因可能是鲍曼不动杆菌的一个重要节点[15-16],尚需进一步研究。
| [1] | 王娜, 贾俐萍, 王莉, 等. 医院耐碳青霉烯类铜绿假单胞菌的流行及相关因素分析[J]. 中国医院药学杂志, 2016, 36(2): 138–140. |
| [2] | 王涛, 王瑞兰. 鲍曼不动杆菌医院感染相关危险因素及预防[J]. 中国感染与化疗杂志, 2015, 15(1): 81–83. |
| [3] | Magiorakos AP, Srinlivasan A, Carey RB. Multi-drug resistant, extensive drug resistantand pan-drug resistant bacteria:an international expert proposal for interim standard definitions for acquired resistance[J]. Clin Microbiol Infect, 2012, 18(3): 268–281. DOI:10.1111/j.1469-0691.2011.03570.x |
| [4] | 王军, 刘勇. 常见非发酵菌的临床分布及耐药性分析[J]. 实用药物与临床, 2016, 19(2): 235–240. |
| [5] | 史尊基, 李超, 胡予丹. 我院常见非发酵菌的临床变化及耐药性分析[J]. 中国当代医药, 2014, 21(24): 187–198. |
| [6] | 任伟, 龙晓玲, 周涛, 等. 儿童铜绿假单胞菌脓毒症14例临床分析[J]. 临床儿科杂志, 2016, 34(9): 674–676. |
| [7] | He S, He H, Chen Y, et al. In vitro and in vivo analysis of anti-microbial agents alone and in combination against multidrug resistant Acinetobacter baumannii[J]. Front Microbiol, 2015, 6: 507–512. |
| [8] | Liu Q, Li W, Du X, et al. Risk and prognostic factors for multidrug-resistant Acinetobacter baumannii complex bacteremia:A retrospective study in a Tertiary Hospital of West China[J]. PLoS One, 2015, 10(6): e0130701. DOI:10.1371/journal.pone.0130701 |
| [9] | 陈越, 孙景勇, 倪语星, 等. 2012年中国CHINET铜绿假单胞菌耐药性监测[J]. 中国感染与化疗杂志, 2015, 15(3): 199–203. |
| [10] | Cholley P, Hocquet D, Alauzet C, et al. Hospital outbreak of Pseudomonas aeruginosa producing extended-spectrumy oxacillinase OXA-19[J]. J Med Microbiol, 2010, 59(Pt7): 866–869. |
| [11] | 蒋月婷, 麦嘉玲, 陈定强, 等. 耐碳青霉烯铜绿假单胞菌的耐药基因检测及分析[J]. 中国抗生素杂志, 2016, 41(7): 552–556. |
| [12] | 许亚丰, 耿先龙, 王春新, 等. 多重耐药鲍曼不动杆菌β-内酰胺酶、膜孔蛋白及β-内酰胺类靶位编码基因研究[J]. 中国抗生素杂志, 2014, 39(1): 58–64. |
| [13] | 陈秀荣, 王江南, 朱小燕, 等. ICU患者感染鲍曼不动杆菌对碳青霉烯类抗生素的耐药性及相关耐药基因分析[J]. 中国病原生物学杂志, 2015, 10(8): 759–762. |
| [14] | 李永丽, 陈艺升, 汪雅萍, 等. 重症监护病房鲍曼不动杆菌耐药性与分子流行病学研究[J]. 中国感染与化疗杂志, 2015, 15(6): 583–587. |
| [15] | 邓正华, 温先勇, 刘靳波, 等. LAMP法检测耐碳青霉烯类鲍曼不动杆菌OXA-23基因的研究[J]. 国际检验医学杂志, 2015, 36(4): 513–515. |
| [16] | 邓德耀, 袁文丽, 吴迪, 等. 重症监护病房耐碳青霉烯类鲍曼不动杆菌碳青霉烯酶基因型的检测[J]. 昆明医科大学学报, 2015, 36(7): 62–66. |
 2017, Vol. 43
2017, Vol. 43


