扩展功能
文章信息
- 徐吉光, 李宇宁
- XU Jiguang, LI Yuning
- 叶酸联合维生素B12对高同型半胱氨酸血症引起大鼠动脉粥样硬化的治疗作用
- Therapeutic effect of folic acid combined with vitamin B12 on atherosclerosis induced by hyperhomocysteinemia in rats
- 吉林大学学报(医学版), 2017, 43(05): 943-947
- Journal of Jilin University (Medicine Edition), 2017, 43(05): 943-947
- 10.13481/j.1671-587x.20170516
-
文章历史
- 收稿日期: 2017-01-11
2. 兰州大学第一医院小儿科, 甘肃 兰州 730700
2. Department of Pediatrics, First Hospital, Lanzhou University, Lanzhou 730700, China
动脉粥样硬化是心脑血管系统疾病的主要原因,其危险因素包括吸烟、糖尿病、高血脂和高血压等,同型半胱氨酸(homocysteine,Hcy)是能量代谢和甲基化反应的中间产物,Hcy血症的毒性作用机制比较复杂,是动脉粥样硬化的独立危险因素,可以引起和促进动脉粥样硬化的发生发展[1-2]。国内外研究[3-4]显示:叶酸和维生素B12是Hcy代谢过程的辅因子,摄入蛋氨酸过多,叶酸、维生素B12缺乏及其代谢所需酶活性下降或者基因缺陷等可引起Hcy水平升高。动脉粥样硬化病理过程复杂,包括大量脂质及多种炎症细胞的参与,主要机制包括脂质的沉积、炎症反应、血管平滑肌细胞增殖和迁移、内皮损伤,动脉发生病变即为细胞外基质重建。有实验研究[5]证明基质金属蛋白酶9(matrix metalloproteinase-9,MMP-9) 在动脉粥样硬化血管重构及斑块破裂、稳定方面发挥重要作用。动脉粥样硬化发病过程的重要标志为大量单核细胞在内皮下间隙聚集,单核细胞的迁移主要有趋化因子介导,CC趋化因子配体5(CC chemokine ligand 5,CCL5) 具有参与炎症反应、激活淋巴细胞、对多种细胞的趋化作用,CC趋化因子受体5(CC chemokine receptor 5,CCR5) 为CCL5受体,与CCL5具有较高的亲和力,在动脉粥样硬化的发病中具有重要作用[6]。但关于叶酸联合维生素B12应用于动脉粥样硬化的研究尚不多见。因此,本研究采用Hcy建立动脉粥样硬化大鼠模型,探讨叶酸联合维生素B12对动脉粥样硬化大鼠血清Hcy和MMP-9及胸主动脉平滑肌细胞中CCR5和CCL5表达的影响。
1 材料与方法 1.1 实验动物、主要试剂和仪器清洁级、健康、雄性Wistar大鼠33只、4~8周龄、体质量100~140 g,购自北京维通利华实验动物技术有限公司,动物合格证号:SCXK-(京)2006-0009。维生素B12片(利丰华瑞制药有限公司),叶酸片(飞鹰制药有限公司),MMP-9 ELISA试剂盒、Hcy ELISA试剂盒、蛋氨酸、逆转录-聚合酶链反应(RT-PCR)试剂盒和即用型免疫组织化学试剂盒(美国Sigma公司),CCR5兔抗鼠多克隆抗体和CCL5兔抗鼠多克隆抗体(博士德生物工程有限公司)。CX7型全自动生化检测仪(美国Beakman公司),Blofuge28RS型高速冷冻离心机(德国贺利氏集团公司),RM2235型全自动轮转石蜡切片机(德国徕卡公司),FLUOR plus型多功能酶标仪(瑞典Tecan公司),Stepone Plus型荧光定量PCR仪(美国ABI公司),AEL-160电子分析天平(日本岛津公司)。
1.2 实验大鼠模型制备及分组33只大鼠按照随机数字法分为对照组、模型组(高蛋氨酸组)和治疗组(叶酸+维生素B12治疗组)。对照组大鼠给予普通饲料喂养;模型组大鼠在普通饲料喂养的基础上添加蛋氨酸(20 g·kg-1)喂养;治疗组大鼠在普通饲料+蛋氨酸喂养的基础上每天给予叶酸和维生素B12(0.5 mg叶酸和25 μg维生素B12溶于2 mL生理盐水)灌胃;对照组和模型组大鼠给予等量生理盐水灌胃,每组大鼠喂养2个月。
1.3 标本采集喂养2个月末,各组大鼠行腹腔注射麻醉,开腹,剥离、暴露腹主动脉,抽取腹主动脉血3 mL离心(3 000 r·min-1)分离血清,用于ELISA法检测;处死大鼠,取颈总动脉,用于HE染色、免疫组织化学染色和RT-PCR实验。
1.4 HE染色将各组大鼠的胸主动脉置入甲醛中固定24 h,进行石蜡包埋,切成厚5 μm切片,常规HE染色,观察各组大鼠胸主动脉的病理组织形态表现。
1.5 各组大鼠血清Hcy和MMP-9水平检测采用ELISA法检测血清Hcy和MMP-9水平,测定450 nm波长处吸光度(A)值。
1.6 免疫组织化学染色检测各组大鼠胸主动脉平滑肌细胞中CCR5和CCL5蛋白表达水平将石蜡切片切成5 μm厚切片,进行即用型免疫组织化学染色,以CCR5兔抗鼠多克隆抗体和CCL5兔抗鼠多克隆抗体为一抗,阴性对照以PBS液代替一抗,胞浆中出现棕褐色或者棕黄色颗粒为CCR5和CCL5蛋白表达阳性,随机测量5个高倍视野胸主动脉平滑肌细胞中CCR5和CCL5的平均灰度值,灰度值越小表示阳性表达越强,灰度值越大表示阳性表达越弱。
1.7 RT-PCR法检测各组大鼠胸主动脉平滑肌细胞中CCR5和CCL5 mRNA表达水平提取各组大鼠胸主动脉平滑肌细胞中总RNA,采用RT-PCR法测定胸主动脉平滑肌细胞中CCR5和CCL5 mRNA表达水平,采用SYNGENE公司的图像分析系统分析CCR5和CCL5 mRNA的表达情况,计算CCR5和CCL5 mRNA相对表达水平,CCR5和CCL5 mRNA相对表达水平=目的基因扩增条带积分A值/β-actin基因扩增条带积分A值。
1.8 统计学分析采用SPSS 20.0软件进行统计学分析。各组大鼠血清Hcy和MMP-9水平、胸主动脉平滑肌细胞中CCR5和CCL5蛋白和mRNA表达水平均以x±s表示,各组间样本均数比较采用单因素方差分析,组内两两比较采用LSD检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 HE染色观察大鼠胸主动脉病理组织形态表现对照组大鼠胸主动脉中膜平滑肌细胞排列整齐,内弹力膜完整,内皮细胞连续;模型组大鼠胸主动脉中膜平滑肌细胞紊乱疏松,内膜损伤,内膜下有泡沫细胞形成,内皮细胞坏死脱落;治疗组大鼠胸主动脉病理学变化介于对照组和模型组之间。见图 1(插页五)。
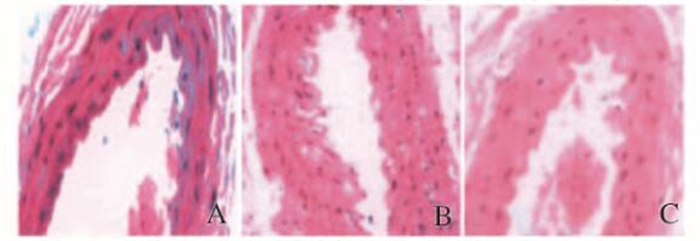
|
| A:Control group; B:Model group; C:Tteatment group. 图 1 各组大鼠胸主动脉HE染色结果(×200) Figure 1 HE staining results of thoracic aorta of rats in various groups(×200) |
|
|
模型组和治疗组大鼠血清Hcy和MMP-9水平高于对照组(P < 0.05),治疗组大鼠血清Hcy和MMP-9水平低于模型组(P < 0.05)。见表 1
| (n=11, x±s) | ||
| Group | Hcy[cB/(μmol·L-1)] | MMP-9[ρB/(μg·L-1)] |
| Control | 11.57±4.12 | 4.57±0.75 |
| Model | 21.24±4.21* | 6.64±0.87* |
| Treatment | 15.63±4.17*△ | 5.64±0.71*△ |
| *P < 0.05 compared with control group;△P < 0.05 compared with model group. | ||
模型组和治疗组大鼠胸主动脉平滑肌细胞中CCR5和CCL5蛋白表达灰度值低于对照组(P < 0.05),CCR5和CCL5 mRNA相对表达水平高于对照组(P < 0.05);治疗组大鼠胸主动脉平滑肌细胞中CCR5和CCL5蛋白表达灰度值高于模型组(P < 0.05),CCR5和CCL5 mRNA相对表达水平低于模型组(P < 0.05)。见表 2。
| (n=11, x±s) | ||||
| Group | CCR5 protein | CCL5 protein | CCR5 mRNA | CCL5 mRNA |
| Control | 144.35±9.86 | 142.31±12.54 | 1.03±0.22 | 0.44±0.02 |
| Model | 123.24±9.77* | 111.46±9.78* | 2.04±0.32* | 0.95±0.02* |
| Treatment | 33.25±8.43*△ | 126.47±10.89*△ | 1.57±0.36*△ | 0.75±0.01*△ |
| *P < 0.05 compared with comtrol group;△P < 0.05 compared with model group. | ||||
动脉粥样硬化是一种由血管平滑肌细胞、淋巴细胞、单核-巨噬细胞、脂质和血管壁内细胞等多种因素共同参与的一种慢性炎症过程。Hcy为食物蛋氨酸衍生物,是甲基化和能量代谢的中间产物。在正常情况下,Hcy释放到细胞外的量较少,血浆中Hcy的浓度是反应Hcy的代谢和生成的指标[7-9],当机体叶酸和维生素B12等辅助因子缺少或代谢所需酶的活性降低时,血浆中Hcy的浓度升高[10],高Hcy与动脉粥样硬化之间的关系比较密切。本研究给予大鼠高蛋氨酸饮食,HE染色结果显示:高蛋氨酸饮食大鼠胸主动脉中膜平滑肌细胞紊乱疏松,内膜损伤,内膜下有泡沫细胞形成,内皮细胞坏死脱落,血清中Hcy水平明显升高,表明高蛋氨酸饮食可引起高Hcy血症的发生,高蛋氨酸饮食大鼠动脉内皮下有泡沫细胞出现,泡沫细胞为动脉粥样硬化病程早期的主要病理学,因此认为高蛋氨酸饮食引起的高Hcy血症可引起动脉粥样硬化的发生。
基质金属蛋白酶(MMPs)是细胞外基质的重要降解辅酶,参与多种病理过程,MMP-9是MMPs家族的重要成员之一,为降解细胞外分泌的主要介质,由平滑肌细胞、血管内皮细胞和单核细胞等多种细胞分泌,在炎症应答时多种细胞因子可以引起MMP-9水平升高[11-12]。在炎症反应过程中,CCL5对多种细胞具有趋化作用,调节细胞的分化和生长,调控炎症反应,激活淋巴细胞等作用[13]。CCR5为CCL5的高亲和力受体,主要在巨噬细胞、T细胞、内皮细胞和平滑肌细胞等细胞中高表达,这些细胞和动脉粥样硬化病变关系密切,T细胞和巨噬细胞等炎症细胞表达的CCR5与CCL5结合,介导炎性细胞的募集和浸润[14-15]。本研究结果显示:模型组大鼠血清MMP-9水平高于对照组,胸主动脉平滑肌细胞中CCR5和CCL5蛋白灰度值低于对照组,大鼠胸主动脉平滑肌细胞中CCR5和CCL5 mRNA相对表达水平高于对照组,表明动脉粥样硬化的发生与血清MMP-9水平、胸主动脉平滑肌细胞中CCR5和CCL5蛋白表达水平升高有关,血清MMP-9水平、胸主动脉平滑肌细胞中CCR5和CCL5蛋白表达水平升高参与动脉粥样硬化形成过程中的炎症反应。
叶酸对Hcy的代谢过程具有决定性作用,在甲硫氨酸循环中使Hcy甲基化生成甲硫氨酸,从而减少Hcy的生成,降低血清Hcy水平;维生素B12可以为Hcy提供甲基使其生成蛋氨酸,叶酸联合维生素B12应用可以提高叶酸的利用率,加强叶酸在血清Hcy的效果[16-17]。本研究结果显示:对动脉粥样硬化大鼠进行叶酸联合维生素B12治疗,可以改善胸主动脉血管的动脉粥样硬化情况,降低血清Hcy水平和血清MMP-9水平以及胸主动脉平滑肌细胞中CCR5和CCL5蛋白表达水平,考虑叶酸联合维生素B12治疗可能通过降低血清Hcy水平和胸主动脉平滑肌细胞中CCR5和CCL5蛋白水平对动脉粥样硬化发挥治疗作用。
| [1] | Emeksiz HC, Serdaroglu A, Biberoglu G, et al. Assessment of atherosclerosis risk due to the homocysteine-asymmetric dimethylarginine-nitric oxide cascade in children taking antiepileptic drugs[J]. Seizure, 2013, 22(2): 124–127. DOI:10.1016/j.seizure.2012.11.007 |
| [2] | 刘睦胜. 血清Hcy及UA水平与老年脑梗死患者头颈部动脉粥样硬化及狭窄的关系[J]. 中国老年学杂志, 2014, 34(23): 6758–6759. DOI:10.3969/j.issn.1005-9202.2014.23.102 |
| [3] | Naseri M, Sarvari GR, Esmaeeli M, et al. High doses of oral folate and sublingual vitamin B12 in dialysis patients with hyperhomocysteinemia[J]. J Renal Inj Prev, 2016, 5(3): 134–139. DOI:10.15171/jrip.2016.28 |
| [4] | 邓远琼, 邓远琪, 刘伯胜, 等. 高血压及叶酸、维生素B12干预对脑梗死患者血管内皮功能和血同型半胱氨酸、一氧化氮水平的影响[J]. 临床神经病学杂志, 2013, 26(6): 411–414. |
| [5] | Usmanova ZA, Rosykhodzhaeva GA. Interrelationship of matrix metalloproteinase-9 and its tissue inhibitor-1 and blood lipid profile of patients with carotid atherosclerosis[J]. Kardiologiia, 2015, 55(9): 57–58. |
| [6] | Golbus JR, Stitziel NO, Zhao W, et al. Common and rare genetic variation in CCR2, CCR5, or CX3CR1 and risk of atherosclerotic coronary heart disease and glucometabolic traits[J]. Circ Cardiovasc Genet, 2016, 9(3): 250–258. DOI:10.1161/CIRCGENETICS.115.001374 |
| [7] | Fang P, Zhang D, Cheng Z, et al. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis[J]. Diabetes, 2014, 63(12): 4275–4290. DOI:10.2337/db14-0809 |
| [8] | Zhang D, Wen X, Wu W, et al. Homocysteine-related hTERT DNA demethylation contributes to shortened leukocyte telomere length in atherosclerosis[J]. Atherosclerosis, 2013, 231(1): 173–179. DOI:10.1016/j.atherosclerosis.2013.08.029 |
| [9] | 王三敏, 伏兵, 佘瑞芳, 等. 血清同型半胱氨酸和巨噬细胞移动抑制因子水平与颈动脉粥样硬化相关性研究[J]. 重庆医学, 2014, 43(2): 182–184. |
| [10] | Višekruna I, Rumbak I, Samarin IR, et al. Homocysteine levels show significant differences among mediterranean dietary quality index variables compared to folate and vitamin B(12) status in women[J]. Int J Vitam Nutr Res, 2015, 85(3/4): 202–210. |
| [11] | Jin ZX, Xiong Q, Jia F, et al. Investigation of RNA interference suppression of matrix metalloproteinase-9 in mouse model of atherosclerosis[J]. Int J Clin Exp Med, 2015, 8(4): 5272–5278. |
| [12] | 吴硕, 赵延新. 脑梗死患者颈动脉粥样硬化性斑块与CD147 MMP9 TIMP-1相关性[J]. 浙江临床医学, 2015, 17(11): 1965–1966. |
| [13] | Marques RE, Guabiraba R, Russo RC, et al. Targeting CCL5 in inflammation[J]. Expert Opin Ther Targets, 2013, 17(12): 1439–1460. DOI:10.1517/14728222.2013.837886 |
| [14] | Cipriani S, Francisci D, Mencarelli A, et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice[J]. Circulation, 2013, 127(21): 2114–2124. DOI:10.1161/CIRCULATIONAHA.113.001278 |
| [15] | 李丹娜, 张金盈, 李蒙, 等. 冠心病患者血清CC类趋化因子配体5与单核细胞趋化蛋白-1的水平及意义[J]. 中国实用医刊, 2009, 36(5): 54–55. |
| [16] | Thomas P, Fenech M. Buccal cytome biomarkers and their association with plasma folate, vitamin B12 and homocysteine in Alzheimer's disease[J]. J Nutrigenet Nutrigenomics, 2015, 8(2): 57–69. DOI:10.1159/000435784 |
| [17] | 隋利军, 桑建, 朝亚, 等. 叶酸、维生素B12对老年高血压患者血浆同型半胱氨酸水平及血管内皮功能的影响[J]. 中国生化药物杂志, 2015, 36(1): 125–127. |
 2017, Vol. 43
2017, Vol. 43


