引用本文 | 0 |
周世平, 徐红, 王琇, 李蓥. 内镜黏膜下剥离术治疗食管颗粒细胞瘤2例报告并文献复习[J]. 吉林大学学报(医学版), 2017, 43(05): 1034-1036.

ZHOU Shiping, XU Hong, WANG Xiu, LI Ying. Endoscopic submucosal dissection in treatment of esophageal granular cell tumor:A report of 2 cases and literature review[J]. Journal of Jilin University (Medicine Edition), 2017, 43(05): 1034-1036.

内镜黏膜下剥离术治疗食管颗粒细胞瘤2例报告并文献复习
吉林大学第一医院胃肠内科内镜中心, 吉林 长春 130021
收稿日期: 2017-04-08
基金项目: 吉林省科技厅白求恩专项基金资助课题(20160101134JC)
作者简介: 周世平(1974-), 女, 吉林省长春市人, 主要从事消化内镜诊治方面的研究。
[摘要]:
目的:
报道2例内镜黏膜下剥离术(ESD)治疗食管颗粒细胞瘤的病例,以提高对该病的临床认识。方法:
回顾性分析2例患者的胃镜和超声胃镜结果,初步诊断为食管黏膜下隆起,遂行ESD完整切除肿瘤。首先标记病变边界,然后向黏膜下层注射透明质酸使病变充分隆起,沿标记点外缘将黏膜层环周切开,沿黏膜下层逐渐剥离病变至整块剥离,最后进行必要的止血及钛夹闭合创面。结果:
术后病理显示,细胞呈多边形或梭形,胞质丰富且含有嗜双色性颗粒状物质,细胞核小;免疫组织化学显示,S-100(+),NSE(+)/(-),CK(-),SMA(-),CD117(-),desmin(-),证实为食管颗粒细胞瘤,患者术后顺利恢复,术后16个月复查,2例患者均无局部复发及转移。结论:
ESD是一种治疗食管颗粒细胞瘤安全有效的方法,患者术后预后良好。
关键词: 食管颗粒细胞瘤 超声胃镜 免疫组织化学 内镜下黏膜切除术 内镜黏膜下剥离术
Endoscopic submucosal dissection in treatment of esophageal granular cell tumor:A report of 2 cases and literature review
ZHOU Shiping,
XU Hong

,
WANG Xiu,
LI Ying

Gastroenterology and Endoscopic Center, First Hospital, Jilin University, Changchun 130021, China
[Abstract]:
Objective:
To report two cases of esophageal granular cell tumor treated by endoscopic submucosal dissection, and to improve the clinical awareness of the disease.
Methods:
The results of endoscope and ultrasonography endoscope of 2 cases were retrospectively analyzed. A preliminary diagnosis of the 2 cases was esophageal submucosal protruded lesion. The patients underwent endoscopic submucosal dissection and the tumors were removed completely. Firstly, the lesion border was marked and then hyaluronic acid was injected into submucosa to uplift the lesion sufficiently. Secondly, the mucosa was circumferentially incised along the marked points, and then the lesion was dissected along the submucosal layer gradually to the whole. Finally, necessary hemostatic was undertaken and the wound was clipped with titanium.
Results:
The pathological results after operation represented that the cells were polygonal or fusiform, rich in cytoplasm and had a dichromatic granular substance, and the nuclei were small.The immunohistochemical results showed S-100(+), NSE(+)/(-), CK(-), SMA(-), CD117(-), desmin(-) and proved that 2 cases of tumors were esophageal granular cell tumor. Two patients recovered smoothly after operation. They underwent endoscopic examination 16 months after operation, and no local recurrence or metastasis was founded.
Conclusion:
Endoscopic submucosal dissection is a safe and effective treatment of esophageal granular cell tumor, and the postoperative prognosis is good.
Key words:
esophageal granular cell tumor
endoscopic ultrasonography
immunohistochemistry
endoscopic mucosa resection
endoscopic submucosal dissection
颗粒细胞瘤(granular cell tumor,GCT)是一种罕见的软组织肿瘤,起源于Schwann细胞,可发生于身体各个部位,消化道仅占4%~6%,其中食管约占2%,形态不规则,一般呈孤立性结节状[1]。食管CGT多呈良性临床经过,生长缓慢,但也有恶变潜能,新近文献[2]不乏关于恶性食管GCT的报道。对GCT的治疗既往以外科手术为主,但随着内镜技术的发展,内镜黏膜下剥离术(endoscopic submucosal dissection,ESD)也开始应用于该病的治疗。本文作者总结本科室应用ESD完整切除食管GCT的2例病例,现将结果报道如下。
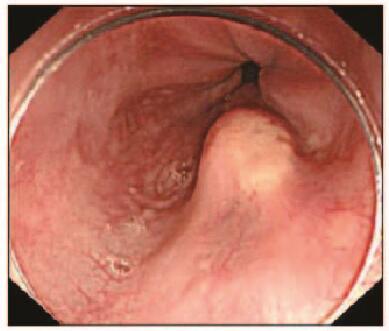
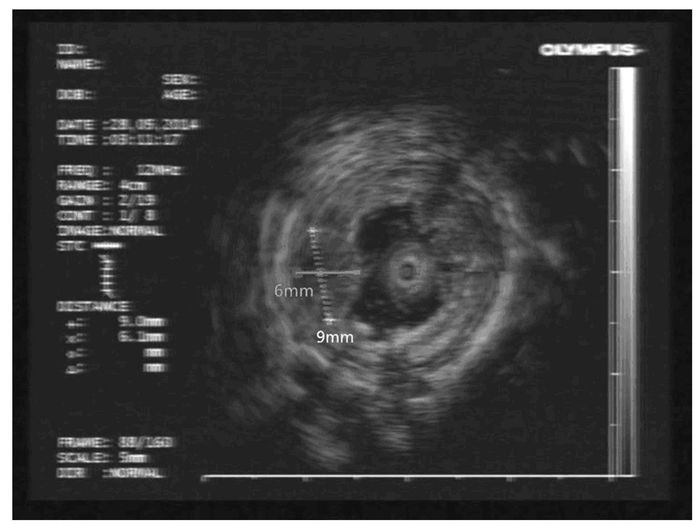
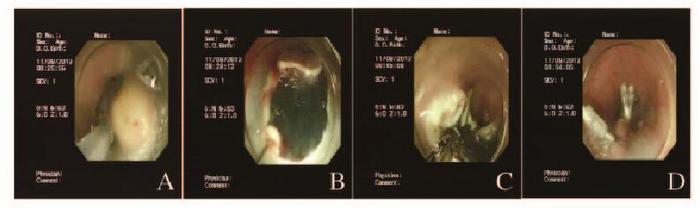
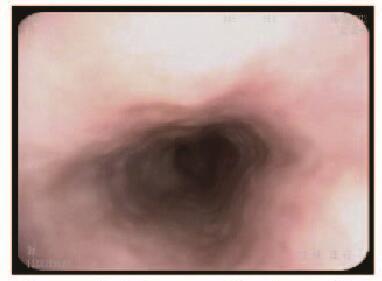
1 临床资料 患者1,男性,51岁,因反复烧心、返酸10余年,于2015年11月就诊于本院胃肠内科内镜中心。胃镜:食管距门齿约36 cm处见一大小约0.8 cm×1.0 cm黏膜隆起,表面尚光滑,部分呈黄白色外观。超声胃镜(EUS):隆起处扫描可见一低回声病灶,考虑位于管壁黏膜下层,内部回声尚均一,境界尚清,切面大小为9 mm×6 mm。见图 1(插页六)和2。
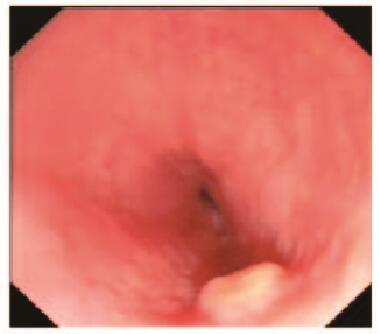
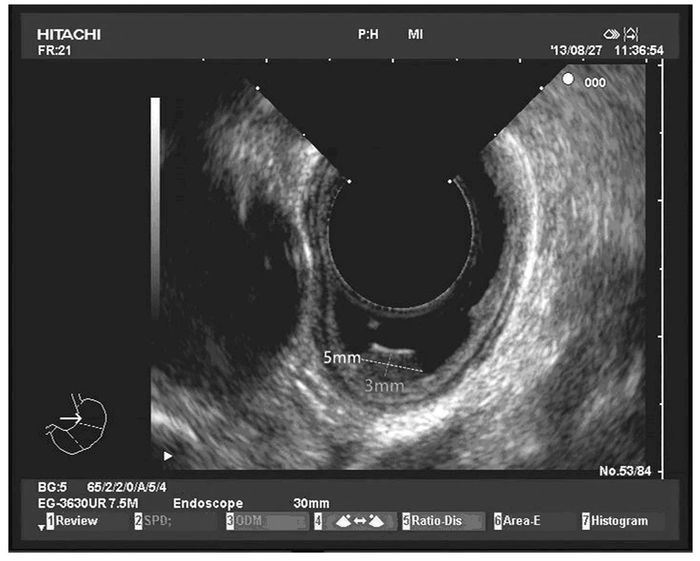
患者2,女性,34岁,因上腹部胀满,伴恶心、嗳气3年,于2016年2月就诊于本院胃肠内科内镜中心。胃镜:食管距门齿约35 cm处见一大小约0.6 cm×0.6 cm黏膜隆起,表面光滑,色黄白,无糜烂及坏死。EUS:病变似位于黏膜层,以低回声为主,顶端呈高回声,截面大小约5 mm ×3 mm,未见明显血流信号。见图 3(插页六)和4。
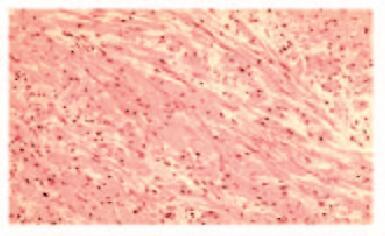
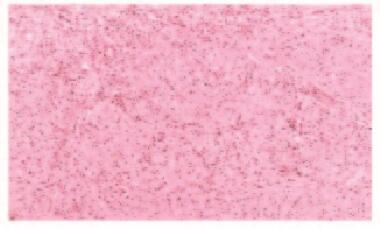
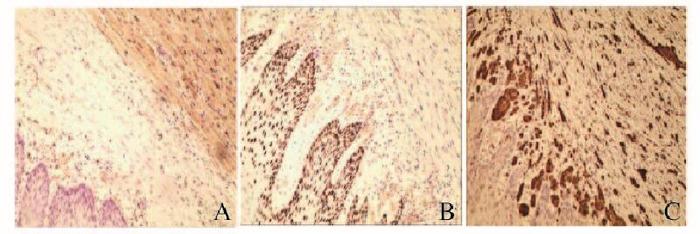
2例患者均行ESD治疗,术后创面无出血,瘤体切除完整(图 5,见插页六)。术后病理显示:肿瘤细胞呈紧密的巢状或片状,细胞呈圆形、多边形或梭形,细胞质丰富且含有多量嗜双色性颗粒状物质,细胞核小、圆而居中(图 6和7,见插页六)。患者1免疫组织化学检测结果显示:S-100(+),神经特异性烯醇化酶(NSE,-),角蛋白(CK, -),增殖指数(Ki-67, +)<1%,平滑肌肌动蛋白(SMA, -),CD68(+)灶,CD34(+)灶, CD117(-),P53(-),抗黑素瘤特异性单抗(HMB45, -),肌动蛋白(Actin, -)。患者2免疫组织化学检测结果显示:S-100(+),NSE(+),CK(-),Ki-67(+)<2%,SMA(-),肌间蛋白(desmin, -),波形蛋白(Vimentin, +)(图 8,见插页六)。2例患者均于ESD术后16个月复查胃镜,均无局部复发及转移(图 9和10,见插页六)。
2 讨论 食管GCT可发生于任何年龄,多见于40~60岁,女性多于男性,主要发生于食管中下段。本组2例患者病变均位于食管中下段,与文献报道相符[3]。食管GCT生长缓慢,无明显临床症状,常在内镜检查时偶然发现。内镜检查是诊断本病的主要方法,内镜下食管GCT多表现为黏膜下隆起,肿瘤直径<2 cm,呈结节状或无蒂息肉状,表面呈淡黄色,可伴有浅糜烂,少数可见局部呈环形狭窄。Kim等[4]报道:超声内镜显示食管GCT多位于黏膜肌层或固有肌层,均质,低回声,边界欠清。
病理检查和免疫组织化学检测是诊断本病的金标准。光镜下显示细胞呈多边形、梭形和卵圆形等;胞体较大,胞浆丰富,含有较多大小相似、分布均匀的嗜酸颗粒;核小且大小一致,很难找到核分裂相。免疫组织化学检测结果显示:GCT肿瘤细胞表达S-100,部分可表达NSE, 但不表达肌源性抗体SMA和CD34、CD117和desmin,最近发现的另一个新的神经标记物nestin也在GCT中表达, 特别是在胃肠道的GCT中表达[5-6]。
食管GCT绝大多数为良性,恶性者少于2%[7],表现为瘤体复发、生长快、直径大于4 cm、呈浸润性生长和出现多处转移等[8]。良恶性食管GCT在临床病理学上高度相似,目前认为转移是恶性食管GCT的唯一证据。通常认为有无转移、手术完整切除后能否复发是鉴别良、恶性食管GCT的临床标准[9]。
针对食管GCT的治疗方式目前尚不统一,主要有内镜动态随诊、内镜切除和外科切除。文献[10]显示:直径小于1 cm且无临床症状的患者可以采取内镜随诊。随着消化内镜技术的发展,内镜下切除食管GCT因其创伤小、术后感染风险低、不影响患者生活质量等优点而被广泛应用于临床。主要手段为内镜下黏膜切除术(EMR)[11]和ESD,但EMR不适合切除直径超过1 cm的病变,这是因为食管GCT不局限于黏膜层,常常侵及黏膜下层,EMR很可能切除不完整或引起穿孔等严重并发症,Zhong等[12]曾报道1例EMR切除直径13 mm的食管GCT不成功的案例。ESD术首先剥离病变的黏膜下层,再围绕病变行全周的黏膜切除,其优势在于即使病变较大,也能达到大体上及组织学上的完全切除[13]。目前ESD技术已被广泛应用于消化道早癌的治疗,也被用于切除黏膜下肿瘤[14]。与传统的EMR比较,ESD能增加整块切除和完整切除的概率,进而减少局部复发率[15]。文献[16]报道:GCT患者行ESD术,整块切除率为92.9%。由此可见,ESD治疗食管GCT具有安全、有效和创伤小等优势。出血和穿孔是食管GCT行ESD术的2个主要并发症。本组2例病例并未出现任何并发症。本中心减少并发症的措施包括:① 保持术野清晰,任何操作都在直视下进行;② 对所见血管行预防性止血;③ 术中精确把握手术刀的长度和角度;④ 病变切除后, 对整个创面行热活检钳电凝或氩气喷凝(APC)。这些措施可以减少迟发性出血并消除残存病变。由于本研究病例数有限及随访时间较短,还需要延长随访时间来进一步评价食管颗粒细胞瘤ESD术的长期疗效。对于肿瘤瘤体较大,已累及肌层,或出现明显梗阻症状时,考虑到此类肿瘤有恶性潜能,应外科手术切除[17],必要时加上区域淋巴结清扫。
综上所述,食管GCT临床罕见,ESD治疗该病完整切除率高,无出血和穿孔等并发症,能达到满意的临床效果。
参考文献
| [1] |
Narra SL, Tombazzi C, Datta V, et al.
Granular cell tumor of the esophagus:report of five cases and review of the literature[J]. Am J Med Sci, 2008, 335(5): 338–341.
DOI:10.1097/MAJ.0b013e3181568197 |
|
| [2] |
Choi SM, Hong SG, Kang SM, et al.
A case of malignant granular cell tumor in the sigmoid colon[J]. Clin Endosc, 2014, 47(2): 197–200.
DOI:10.5946/ce.2014.47.2.197 |
|
| [3] |
邹兰英, 赵会传, 王晓艳, 等.
食管颗粒细胞瘤七例临床病理特征分析[J]. 临床内科杂志, 2015, 32(7): 473–475.
|
|
| [4] |
Kim DU, Kim GH, Ryu DY, et al.
Endosonographic features of esophageal granular cell tumors using a high-frequeIlcy cathetel probe[J]. Scand J Gastroenterol, 2011(2): 142–147.
|
|
| [5] |
纪小龙.
消化道病理学[M]. 北京: 人民军医出版社,2010: 264.
|
|
| [6] |
胥荣, 李红霞, 范钦和.
食管颗粒细胞瘤4例临床病理分析[J]. 诊断病理学杂志, 2010, 17(2): 114–116.
|
|
| [7] |
Aoyama K, Kamio T, Hirano A, et al.
Granular cell tumors:a report of six cases[J]. World J Surg Oncol, 2012, 10: 204.
DOI:10.1186/1477-7819-10-204 |
|
| [8] |
黄华, 赵金平, 高思海.
食管下段颗粒细胞瘤1例临床报道并文献复习[J]. 华中科技大学学报:医学版, 2010, 39(5): 729–730.
|
|
| [9] |
孙艾茜, 魏志, 刘长江, 等.
内镜下治疗食管颗粒细胞瘤4例临床分析[J]. 现代消化及介入诊疗, 2014, 19(1): 68–69.
|
|
| [10] |
王宁宁, 李异玲, 孙明军.
内镜下治疗食管颗粒细胞瘤一例[J]. 中国医师进修杂志, 2014, 37(31): 76–77.
DOI:10.3760/cma.j.issn.1673-4904.2014.31.028 |
|
| [11] |
许海英, 姜威, 陈刚, 等.
内镜下黏膜切除术治疗食管颗粒细胞瘤二例[J]. 中华胃肠内镜电子杂志, 2014, 1(2): 95–96.
|
|
| [12] |
Zhong N, Katzka DA, Smyrk TC, et al.
Endoscopic diagnosis and resection of esophageal granular cell tumors[J]. Dis Esophagus, 2011, 24(8): 538–543.
DOI:10.1111/des.2011.24.issue-8 |
|
| [13] |
Zhou PH, Yao LQ, Qin XY, et al.
Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors:a retrospective study[J]. Surg Endosc, 2010, 24(10): 2607–2612.
DOI:10.1007/s00464-010-1016-z |
|
| [14] |
Komori K, Akahoshi K, Tanaka Y, et al.
Endoscopic submucosal dissection for esophageal granular cell tumor using the clutch cutter[J]. World J Gastrointest Endosc, 2012, 4(1): 17–21.
DOI:10.4253/wjge.v4.i1.17 |
|
| [15] |
Nie L, Xu G, Wu H, et al.
Granular cell tumor of the esophagus:a clinicopathological study of 31 cases[J]. Int J Clin Exp Pathol, 2014, 7(7): 4000–4007.
|
|
| [16] |
Lu W, Xu MD, Zhou PH.
Endoscopic submucosal dissection of esophageal granular cell tumor[J]. World J Surg Oncol, 2014, 12(1): 221.
DOI:10.1186/1477-7819-12-221 |
|
| [17] |
徐显林, 邹冬梅, 李海军, 等.
窄带成像内镜下诊治食管上段颗粒细胞瘤1例并文献复习[J]. 胃肠病学, 2014, 19(7): 445–446.
|
|

 2017, Vol. 43
2017, Vol. 43


