扩展功能
文章信息
- 刘相良, 纪伟, 李理, 赵颖华, 贾琳, 万志强, 陈晓, 李薇
- LIU Xiangliang, JI Wei, LI Li, ZHAO Yinghua, JIA Lin, WANG Zhiqiang, CHEN Xiao, LI Wei
- 盐酸厄洛替尼片及耐药后化疗联合贝伐珠单抗注射液治疗肺腺癌1例报告并文献复习
- Lung adenocarcinoma treated by Erlotinib HCL Tablets and chemotherapy combined with bevacizumab after drug resistance:A case report and literature review
- 吉林大学学报(医学版), 2017, 43(05): 1019-1024
- Journal of Jilin University (Medicine Edition), 2017, 43(05): 1019-1024
- 10.13481/j.1671-587x.20170530
-
文章历史
- 收稿日期: 2016-12-01
2. 吉林大学中日联谊医院结直肠肛门外科, 吉林 长春 130033
2. Department of Gastrointestinal Colorectal and Anal Surgery, China-Japan Union Hospital, Jilin University, Changchun 130033, China
肺癌已经成为世界范围内癌症相关性死亡的重要因素,约85%的肺癌为非小细胞肺癌(non-small-cell lung carcinoma, NSCLC),其5年总生存率仅为16%[1]。早期肺腺癌治疗以肺叶切除术为主,晚期肺腺癌患者若检测出表皮生长因子受体(epidermal growth factor receptor,EGFR)基因突变型则应用表皮生长因子受体酪氨酸激酶抑制剂(epidermal growth factor receptortyrosine kinaseinhibitors, EGFR-TKIs)作为一线治疗方案[2]。然而,美国国立综合癌症网络(NCCN)指南对于EGFR基因突变型肺腺癌四线化疗后疾病进展的治疗方法未做明确说明,因此临床医生应积极进行临床试验积累有效治疗经验。本文作者报道EGFR基因突变型晚期肺腺癌1例,并探讨患者应用EGFR-TKI的疗效、耐药后经过四线化疗以及采用化疗联合贝伐珠单抗注射液进行五线治疗的临床疗效。
1 临床资料 1.1 一般资料患者,女性,45岁,体检时行肺部CT发现右肺占位就诊于吉林大学第一医院肿瘤中心。患者偶有咳嗽伴少量白色痰,入院前半年有乏力,体质量下降达5 kg;无吸烟史;功能状态评分(performance status, PS)1分。肺部CT显示右肺上叶见软组织密度肿块影,最大层面6.2 cm×7.1 cm;纵隔及右肺门淋巴结肿大,其中4R区纵隔淋巴结1.6 cm×2.0 cm。行全身PET-CT检查显示右肺上叶高代谢肿块,考虑为周围型肺癌;纵隔及右肺门淋巴结转移并伴有多发肝脏转移、多发骨转移并累及左侧髂骨及4、5腰椎椎体(图 1A)。头部MRI检查未见异常。经皮肺穿刺病理报告穿刺组织内可见腺癌浸润;免疫组织化学结果:Ki-67(70%), P63(-), TTF-1(+), CK7(+), CK20(-);EGFR基因检测显示为突变型(19 del)。诊断为右肺腺癌(cT3N2M1 Ⅳ期),肝脏转移癌及多发骨转移癌。
1.2 一线治疗患者口服盐酸厄洛替尼片并针对肺腺癌骨转移灶和肝转移灶分别施用第4腰椎骨水泥置入成型术和经皮肝穿刺射频消融治疗术进行治疗。治疗2个月余后行肺部PET-CT检查见右肺上叶肿块明显缩小及代谢活性下降,评估为疾病稳定(stable disease, SD)。见图 1B。
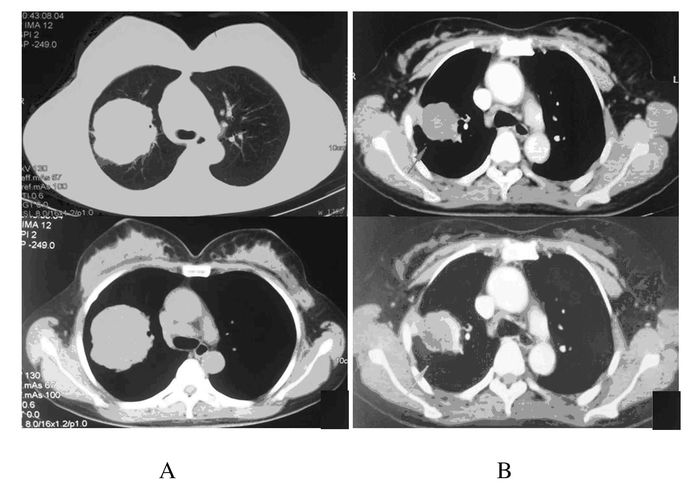
|
| 图 1 肺腺癌患者初诊胸部CT (A)和盐酸厄洛替尼片治疗后胸部CT(B) Figure 1 First diagnosed chest CT (A) and chest CT after treatment of Erlotinib HCL Tablets(B) of patient |
|
|
继续治疗8个月后患者因自感腰痛难止就诊,行腰部CT显示第1腰椎椎弓根处新发转移灶。行腰椎第1、4和5椎体及其附件骨转移癌放疗(治疗剂量为40 Gy/2Gy/20 f),放疗后腰部疼痛明显减轻。化疗5个月后,患者自觉头晕、头痛,伴恶心、呕吐入院,行头部MRI可见颅内发生多个癌转移灶,后行全脑放疗(治疗剂量:40 Gy/2 Gy/20 f局部病灶补量:15 Gy/3 Gy/5 f),放疗后头晕头痛症状完全缓解。
1.4 三线治疗继续治疗2个月后,患者自觉偶有咳嗽和右上腹不适,再次入院。行肺部、腹部CT检查显示:右肺部原发灶有明显增大,左肺转移灶无明显改变(图 2A);肝转移灶体积明显增大并多发(图 3A)。评估为疾病进展(progressive disease, PD), 无病进展期(progressive-free survival, PFS)共计17个月。应用注射用培美曲塞二钠(500 mg·m-2)及注射用卡铂(AUC5 d1) 治疗2个周期后患者复查肺、腹部CT显示肺部原发灶较前略有缩小(图 2B),部分肺内转移瘤较前增大,肝脏转移病灶进展(图 3B),评估为PD。
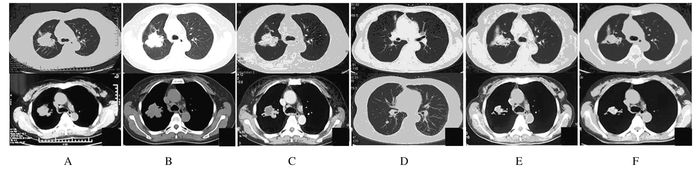
|
| A: Chest CT after 2-month therapy of Erlotinib HCL Tablets; B:Chest CT after 2-round therapy of pemetrexed disodium and carboplatin for injection; C: Chest CT after 2-round therapy of gemcitabine hydrochloride and cisplatin for injection; D: Chest CT after 4-round therapy of gemcitabine hydrochloride and cisplatin for injection; E: Chest CT after 2-round therapy of paclitaxel (albumin bound) for injection; F: Chest CT after 3-round therapy of paclitaxel (albumin bound) for injection. 图 2 肺腺癌患者不同方式治疗后胸部CT Figure 2 Chest CT of patient after different teratments |
|
|
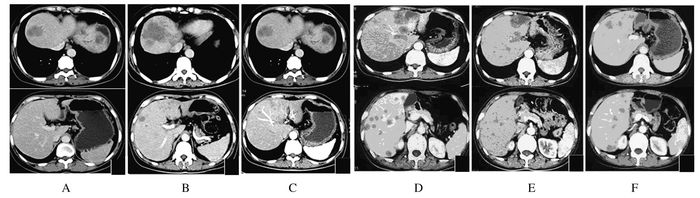
|
| A: Abdomen CT after 2-month therapy of Erlotinib HCL Tablets; B: Abdomen CT after 2-round therapy of pemetrexed disodium and carboplatin for injection; C: Abdomen CT after 2-round therapy of gemcitabine hydrochloride and cisplatin for injection; D: Abdomen CT after 4-round therapy of gemcitabine hydrochloride and cisplatin for injection; E: Abdomen CT after 2-round therapy of paclitaxel (albumin bound) for injection; F: Abdomen CT after 3-round therapy of paclitaxel (albumin bound) for injection. 图 3 不同方式治疗后患者的肝部CT Figure 3 Liver CT of a patient after different treatments |
|
|
进展后患者改用注射用盐酸吉西他滨(1.0 g·m-2,d1,d8) 及注射用顺铂(25 mg·m-2,d1,d2,d3) 治疗,2周期后复查肺、腹部CT见肺原发灶较前略有缩小,肺内转移瘤明显缩小(图 2C),肝脏病灶较前好转(图 3C),评估为SD。继续治疗2周期后再次复查肺、腹部CT见肺原发灶较前略有增大(图 2D),肺内转移瘤进展,肝脏转移病灶增多增大(图 3D),注射用盐酸吉西他滨及注射用顺铂治疗PFS评定为4.5个月。
1.6 五线治疗患者换用注射用紫杉醇(白蛋白结合型)(130 mg·m-2, d1, d8) 单药继续治疗,并于2个周期后复查肺、腹部CT见肺原发灶较前略有缩小,肺内转移瘤明显缩小(图 2E),肝脏转移病灶较前减小(图 3E),评估为SD。继续注射用紫杉醇(白蛋白结合型)单药治疗3个周期后复查胸部CT见病灶基本稳定(图 2F),腹部CT见肝脏转移病灶较前增大(图 3F),评估为PD。注射用紫杉醇(白蛋白结合型)单药治疗PFS为4.5个月。患者行肝功检查显示胆红素明显升高(总胆红素304 μmol·L-1,直接胆红素193 μmol·L-1),考虑为疾病进展肿瘤压迫胆管所致。其后,患者在注射用紫杉醇(白蛋白结合型)(110 mg·m-2,d1,d8) 基础上联合贝伐珠单抗注射液(7.5 mg·kg-1,d1) 治疗1个周期后,胆红素水平恢复接近正常(总胆红素70 μmol·L-1,直接胆红素35 μmol·L-1)。继续治疗2个周期,胆红素水平维持稳定,2个月后,复查胆红素再次升高(总胆红素104 μmol·L-1,直接胆红素63 μmol·L-1)。应用注射用紫杉醇(白蛋白结合型)联合贝伐珠单抗注射液治疗PFS达3个月。患者行胆囊穿刺置管引流术,其后胆红素水平持续升高,总胆红素最高达908 μmol·L-1。约2个月后,患者因肝功能衰竭死亡,总生存期(overall survival, OS)评定为32个月。
2 讨论EGFR是表皮生长因子受体(HER)家族的一员,具有酪氨酸激酶活性。突变型EGFR基因是肺癌最常见的驱动基因之一,由突变型EGFR基因编码的EGFR与三磷酸腺苷(ATP)亲和力低而与EGFR-TKIs亲和力高。约40%的肺腺癌可检测到EGFR基因突变[3-5],“亚洲、女性、非吸烟者及肺腺癌”被视为EGFR基因突变型的优势人群,本例患者诊断为右肺腺癌Ⅳ期(cT3N2M1),伴肝脏转移癌及多发骨转移癌,EGFR基因突变型(19del)。给予口服盐酸厄洛替尼片治疗后PFS达10个月,之后患者出现骨、脑及左肺转移癌。转移癌的发生与EGFR-TKIs耐药有关,Zheng等[6]研究表明:约47% EGFR-TKIs耐药的患者循环血液中存在T790MctDNA,并与较差的预后相关,其他可替代途径的激活,如cMET基因突变或扩增[7-8]、PIK3CA基因突变[9]、BRAF基因突变[10]以及肿瘤细胞转化为小细胞型肺癌(small cell lung cancer, SCLC)[9, 11]或上皮间质转化(epithelial-to-mesenchymal transition,EMT)[9]均可以介导肿瘤细胞对EGFR-TKIs耐药。在第3代EGFR-TKIs药物出现之前,化疗是EGFR-TKIs药物耐药后比较常用的治疗手段。注射用培美曲塞二钠通过叶酸途径阻碍嘌呤核苷酸合成,对快速生长的细胞抑制作用更加明显,其作为非鳞状NSCLC二线治疗药物已经被纳入美国临床肿瘤学会(ASCO)Ⅳ期NSCLC临床指南。与注射用盐酸吉西他滨联合注射用顺铂比较,注射用培美曲塞二钠联合注射用顺铂在腺癌治疗方面获得较长的生存期,且血液毒性发生率更低[12]。但该患者在EGFR-TKIs治疗耐药后应用注射用培美曲塞二钠联合注射用顺铂治疗2周期后疾病未得到控制,改服注射用盐酸吉西他滨联合注射用卡铂治疗后疾病缓解,PFS为4.5个月。
该患者疾病再次进展后选择注射用紫杉醇(白蛋白结合型)单药治疗,注射用紫杉醇(白蛋白结合型)通过促进微管蛋白聚合并抑制其解聚,抑制细胞有丝分裂G2/M期。相比于紫杉醇,注射用紫杉醇(白蛋白结合型)免溶剂,其利用白蛋白作为转运蛋白完成药物在肿瘤微环境的分布,避免了由溶剂所致的毒性反应。该患者应用注射用紫杉醇(白蛋白结合型)2个周期治疗后疾病得到明显控制,治疗5个周期后肝脏转移灶增多并增大,患者肝功能检查结果显示胆红素升高,注射用紫杉醇(白蛋白结合型)单药治疗PFS为4.5个月,高胆红素血症使得并发胆管炎的可能性增加[13-14],炎性微环境又可促进肿瘤的生长和转移。
贝伐珠单抗注射液是重组的人源化单克隆IgG1抗体,抑制人类血管内皮生长因子(vascular endothelial growth factor, VEGF)的活性,使肿瘤血管退化。VEGF作为缺氧诱导因子的一种被认为是肿瘤血管生成的基础因素,与肿瘤的转移相关[15]。贝伐珠单抗注射液抑制肿瘤新生血管生成,切断肿瘤细胞生长所需的营养物质和氧的供应,并通过血管正常化改善肿瘤组织高渗状态,改善化疗药物向肿瘤组织内的传送,提高化疗效果。Terasawa等[16]报道1例直肠癌肝转移患者应用贝伐珠单抗注射液(5 mg·kg-1)治疗2个周期后患者胆红素从156 mg·L-1回降到正常水平(8 mg·L-1)。Camacho等[17]报道3例肝转移癌患者应用贝伐珠单抗注射液治疗后胆红素回降至正常水平。本例患者应用注射用紫杉醇(白蛋白结合型)联合贝伐珠单抗注射液为五线治疗方案,再次获得疾病控制(PFS为3个月),考虑抗血管生成药物在化疗药物耐药后通过其抑制肿瘤新生血管,改善肿瘤微环境后延缓肿瘤生长。然而,炎症信号转导通路中的前列腺素、NF-κB家族分子[18]及Toll样受体等非VEGF途径的激活,可作为肿瘤血管生成的替代途径导致肿瘤细胞耐药。该患者肿瘤耐药后疾病继续进展,肝脏转移病灶增大加重胆管压迫,并发胆管炎症致使胆红素水平持续升高,实施胆囊穿刺置管引流术无效后死亡。
该患者历经盐酸厄洛替尼片、注射用培美曲塞二钠联合注射用卡铂、注射用盐酸吉西他滨联合注射用顺铂、注射用紫杉醇(白蛋白结合型)及贝伐珠单抗注射液共五线治疗,OS达32个月,其中盐酸厄洛替尼片治疗阶段PFS达10个月,约占1/3,显示EGFR突变型肺腺癌患者应用EGFR-TKIs治疗的良好效果。但晚期肺腺癌患者EGFR-TKI一线治疗9~13个月后会产生耐药性,成为制约EGFR-TKIs类药物的瓶颈[19-20]。疾病进展后应用化疗药物治疗是目前广泛使用的手段。该患者在多药耐药及并发高胆红素血症后,应用注射用紫杉醇(白蛋白结合型)联合贝伐珠单抗注射液治疗后胆红素水平下降,继续获得3个月的PFS,其中抗血管生成靶向药物贝伐珠单抗注射液对VEGF的抑制作用是药物取得较好治疗效果重要机制。在目前对于EGFR突变型NSCLC四线治疗耐药后尚无明确推荐方案可循的情况下,该病例在肿瘤多线耐药并发高胆红素血症后采用注射用紫杉醇(白蛋白结合型)联合贝伐珠单抗注射液作为五线治疗方案取得较好的临床疗效,本方案可为临床工作提供参考,也提示临床医生应积极探索化疗药物合理应用,优化临床患者的治疗方案,延长患者生存时间,提高患者的生存质量。
| [1] | Bepler G, Begum M, Simon GR. Molecular analysis-based treatment strategies for non-small cell lung cancer[J]. Cancer Control, 2008, 15(2): 130–139. DOI:10.1177/107327480801500205 |
| [2] | 陆舜, 虞永峰, 纪文翔. 2015年肺癌诊疗指南:共识和争议[J]. 解放军医学杂志, 2016, 41(1): 1–6. DOI:10.11855/j.issn.0577-7402.2016.01.01 |
| [3] | Cadranel J, Zalcman G, Sequist L. Genetic profiling and epidermal growth factor receptor-directed therapy in nonsmall cell lung cancer[J]. Eur Respir J, 2011, 37(1): 183–193. DOI:10.1183/09031936.00179409 |
| [4] | Tsujioka H, Yotsumoto F, Shirota K, et al. Emerging strategies for ErbB ligand-based targeted therapy for cancer[J]. Anticancer Res, 2010, 30(8): 3107–3112. |
| [5] | Greenhalgh J, Dwan K, Boland A, et al. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer[J]. Cochrane Database Syst Rev, 2016(5): CD010383. |
| [6] | Zheng D, Ye X, Zhang MZ, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance[J]. Sci Rep, 2016, 12(6): 20913. |
| [7] | Suda K, Murakami I, Katayama T, et al. Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer[J]. Clin Cancer Res, 2010, 16(22): 5489–5498. DOI:10.1158/1078-0432.CCR-10-1371 |
| [8] | Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling[J]. Science, 2007, 316(5827): 1039–1043. DOI:10.1126/science.1141478 |
| [9] | Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors[J]. Sci Transl Med, 2011, 3(75): 75r. |
| [10] | Ohashi K, Sequist LV, Arcila ME, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1[J]. Proc Nat Acad Sci USA, 2012, 109(31): E2127–2133. DOI:10.1073/pnas.1203530109 |
| [11] | Yatabe Y. EGFR mutations and the terminal respiratory unit[J]. Cancer Metastasis Rev, 2010, 29(1): 23–36. DOI:10.1007/s10555-010-9205-8 |
| [12] | Wang L, Wang R, Pan Y, et al. The pemetrexed-containing treatments in the non-small cell lung cancer is-/low thymidylate synthase expression better than +/high thymidylate synthase expression:a meta-analysis[J]. BMC Cancer, 2014, 14: 205. DOI:10.1186/1471-2407-14-205 |
| [13] | Boulay BR, Parepally M. Managing malignant biliary obstruction in pancreas cancer:choosing the appropriate strategy[J]. World J Gastroenterol, 2014, 20(28): 9345–9353. |
| [14] | Vogel A, Kullmann F, Kunzmann V, et al. Patients with advanced pancreatic cancer and hyperbilirubinaemia:review and german expert opinion on treatment with nab-paclitaxel plus gemcitabine[J]. Oncol Res Treat, 2015, 38(11): 596–603. DOI:10.1159/000441310 |
| [15] | Flaherty KT, Hamilton BK, Rosen MA, et al. Phase Ⅰ/Ⅱ trial of imatinib and bevacizumab in patients with advanced melanoma and other advanced cancers[J]. Oncologist, 2015, 20(8): 952–959. DOI:10.1634/theoncologist.2015-0108 |
| [16] | Terasawa T, Koja S, Yasuda K, et al. A case of metastatic colorectal cancer with icterus due to multiple liver metastases treated effectively by FOLFOX plus bevacizumab[J]. Gan To Kagaku Ryoho, 2013, 40(8): 1115–1118. |
| [17] | Camacho LH, Garcia S, Panchal AM, et al. Exploratory study of hepatic arterial infusion oxaliplatin with systemic 5-fluorouracil/bevacizumab in patients with refractory solid tumor and extensive liver metastases[J]. Clin Colorectal Cancer, 2010, 9(5): 311–314. DOI:10.3816/CCC.2010.n.045 |
| [18] | Bauerle KT, Schweppe RE, Lund G, et al. Nuclear factor kappaB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8[J]. J Clin Endocrinol Meta, 2014, 99(8): E1436–1444. DOI:10.1210/jc.2013-3636 |
| [19] | 程刚, 艾斌. 表皮生长因子受体酪氨酸激酶抑制剂耐药后非小细胞肺癌治疗研究进展[J]. 中国肿瘤临床, 2015, 42(19): 942–946. DOI:10.3969/j.issn.1000-8179.2015.19.847 |
| [20] | 袁冬梅, 宋勇. 非小细胞肺癌治疗新时代:免疫治疗[J]. 解放军医学杂志, 2017, 42(6): 483–487. DOI:10.11855/j.issn.0577-7402.2017.06.01 |
 2017, Vol. 43
2017, Vol. 43


