扩展功能
文章信息
- 于秀艳, 张晓伟, 野丽莉, 李铤, 刘晓峰, 吴雪峰
- YU Xiuyan, ZHANG Xiaowei, YE Lili, LI Ting, LIU Xiaofeng, WU Xuefeng
- hMAM联合MMP-9和C-erbB2 mRNA表达检测在乳腺癌外周血微转移诊断中的应用
- Application of detection of expressions of hMAM combined with MMP-9 and C-erbB2 mRNA in peripheral blood in diagnosis of micrometastases of breast cancer
- 吉林大学学报(医学版), 2017, 43(05): 1009-1014
- Journal of Jilin University (Medicine Edition), 2017, 43(05): 1009-1014
- 10.13481/j.1671-587x.20170528
-
文章历史
- 收稿日期: 2017-02-19
人乳腺珠蛋白(human mammaglobin,hMAM)是1996年Watson等在乳腺癌组织中发现的一种具有高特异性的分泌性球蛋白[1-2]。有研究[3]表明:hMAM对于乳腺癌外周血微转移诊断具有重要临床意义。外周血循环中的肿瘤细胞是导致乳腺癌血行转移与复发的重要原因之一,监测外周血中的肿瘤细胞对早期诊断乳腺癌微转移具有重要意义。在国内外研究中,外周血hMAM mRNA及多种生物标记物联合检测已广泛应用于乳腺癌早期诊断,有常规逆转录PCR法[3-4]、巢式PCR[5]和实时荧光定量PCR[6-7]。hMAM与基质金属蛋白酶9(matrix metallopeptidase 9,MMP-9) 和人表皮生长因子受体2(C-erbB2) mRNA联合检测尚未见相关报道。本研究应用实时荧光定量PCR联合检测乳腺癌患者外周血中hMAM、MMP-9及C-erbB2 mRNA的表达,探讨其在诊断乳腺癌外周血微转移的临床应用价值。
1 资料与方法 1.1 一般资料选择2015年3—12月在吉林省肿瘤医院乳腺治疗中心行手术治疗、术后经病理确诊且病历完整的患者作为研究对象。乳腺癌组74例,患者均为女性,年龄28~79岁,中位年龄49.2岁。乳腺癌分子分型依据2013年StGallen乳腺癌会议国际专家共识,组织学类型按WHO乳腺肿瘤组织学标准分类:浸润性导管癌61例,浸润性小叶癌13例。按照UICC 2003年制订的乳腺癌TNM分期标准:Ⅰ期11例,Ⅱ期17例,Ⅲ期29例,Ⅳ期17例;雌激素受体(ER)阳性19例,孕激素受体(PR)阳性21例,人表皮生长因子受体2(HER2) 阳性18例。乳腺纤维腺瘤组21例,均经术后病理确诊,均为女性,年龄22~75岁,中位年龄47.6岁;健康体检对照组10名,均为女性,年龄24~71岁,中位年龄48.3岁,经胸片和B超检查未发现乳腺相关性疾病。乳腺癌组、乳腺纤维腺瘤组和健康对照组研究对象年龄等一般情况比较差异无统计学意义(P>0.05),具有可比性。
1.2 入组标准及排除标准乳腺癌入组患者结合临床症状、体征,均经病理学确诊为乳腺癌,术前未接受过放、化疗或生物治疗等干预措施,均具有完整的病历资料。排除存在严重心脏及肝肾疾病、自身免疫性疾病、严重并发症、长期嗜酒等情况患者。
1.3 主要试剂和仪器人外周血淋巴细胞分离液(天津市灏洋生物制品科技公司),RNA提取试剂盒(上海Omega公司),逆转录试剂盒和SYBR Green Realtime PCR试剂盒(日本Takara公司),hMAM、MMP-9、C-erbB2和β-actin基因引物(表 1)由宝生物工程(大连)有限公司设计及合成。罗氏LC 480Ⅱ荧光定量PCR仪, 罗氏诊断产品(上海)公司。
| Gene | Primer(5′-3′) | Product size (bp) |
| hMAM | F: ACTCTGAGCAATGTTGAGGTGTTT R: GCAATCCGTAGTTGGTTTCTCAC | 129 |
| MMP-9 | F: TGGGCTGCTGCTTTGCT R: GCCTGTCGGTGAGATTGGTT | 87 |
| C-erbB2 | F: GACGAGACAGAGTACCATGCAGA R: TCACACCATAACTCCACACATCAC | 115 |
| β-actin | F: TGAGCGGGCTACAGCTT R: TCCTTAATGTCACGCACGATTT | 301 |
受试者于清晨空腹采取EDTA抗凝及非抗凝静脉血各3 mL,抗凝血用淋巴细胞分离液分离单个核细胞,提取总RNA用DEPC处理的无菌EP管保存,冻于-80℃待检测。
1.5 实时荧光定量PCR按RNA提取试剂盒说明书提取单个核细胞RNA,NanoDrop 2000C测定RNA含量。取1 μg RNA进行逆转录反应合成cDNA,-80℃保存。总反应体系为25 μL:SYBR Premix Ex Taq Ⅱ 12.5 μL、上下游引物(10 μmol·L-1)各0.5 μL、cDNA 2 μL, 最后加入去离子水9.5 μL。混合后,进行扩增,反应条件为:95℃、30 s, 95℃、5 s, 62℃、30 s, 40个循环。每个样本的每个待测项目均检测3次,取平均值, 记录各样品的Ct值,采用2-ΔΔCt方法计算目的基因的相对表达量[8],以健康对照组为对照。计算公式:标记物基因ΔCt=标记物基因Ct-β-actin Ct;ΔΔCt=乳腺癌或乳腺纤维腺瘤患者ΔCt-健康对照组ΔCt;肿瘤标记物基因的相对表达量为2-ΔΔCt,测定值2-ΔΔCt>2为表达阳性,hMAM联合MMP-9和C-erbB2检测中任意一个指标阳性即为阳性表达。
1.6 统计学分析采用SPSS 17.0统计软件进行统计学分析。乳腺癌组患者外周血中hMAM、MMP-9和C-erbB2 mRNA表达水平以x±s表示,计算采用描述性统计分析及正态性分析,资料均符合正态性分布; 计数资料组间阳性率比较采用χ2检验。检验水准α=0.05。
2 结果 2.1 实时荧光定量PCR标准曲线以浓度为2、2×101、2×102、2×103、2×104和2×105拷贝数·μL-1标准品对数组为横坐标,以扩增CT值为纵坐标绘制标准曲线,线性方程和相关系数:β-actin:y = -3.624 9x + 42.575,R2 = 0.987 8;MMP-9:y = -3.287 1x + 43.643,R2 =0.986 4;C-erbB2:y =-3.964 3x + 41.203,R2 = 0.996 2;hMAM:y =-3.262 9x + 44.953,R2 = 0.989 7。结果显示其线性良好(图 1A)。
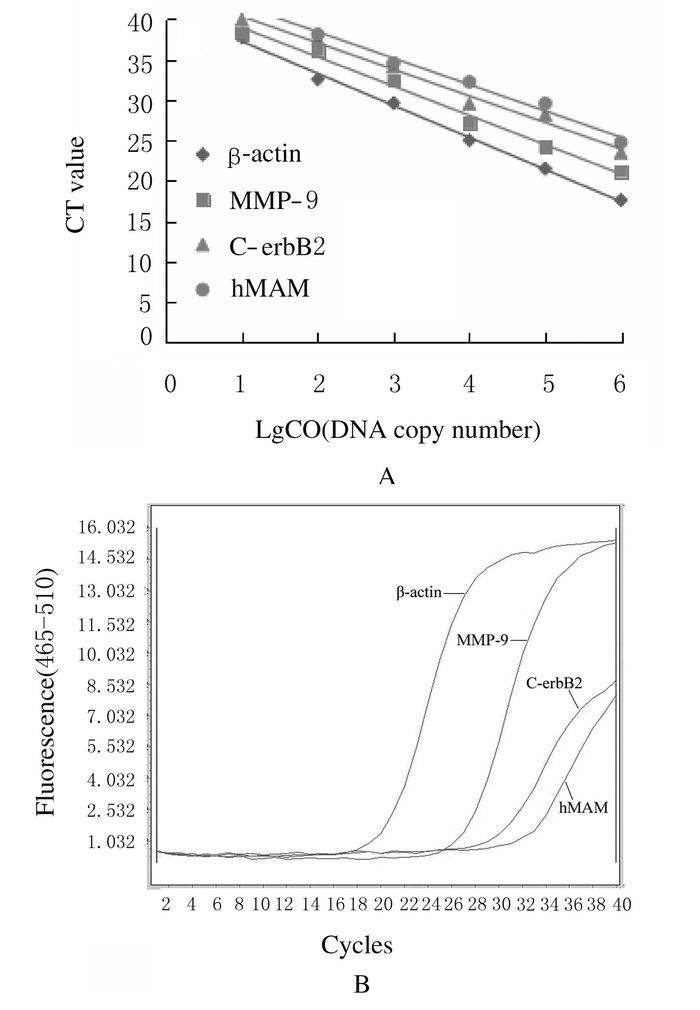
|
| CO:Concentration of starting templates. 图 1 β-actin、MMP-9、C-erbB2和hMAM mRNA PCR标准曲线(A)和扩增曲线(B) Figure 1 PCR standard curves (A) and amplification curves (B) of β-actin (control), MMP-9, C-erbB2 and hMAM mRNA |
|
|
MMP-9、C-erbB2和hMAM mRNA在乳腺癌患者外周血中均有表达(图 1B),其表达水平分别为1.91±1.24、5.63±3.18和8.24±2.72。
2.3 乳腺癌患者外周血中MMP-9、C-erbB2和hMAM mRNA表达阳性率与病理特征的关系随着TNM分期恶性程度增加,乳腺癌患者外周血中MMP-9、C-erbB2和hMAM mRNA阳性表达率增加,但阳性表达率比较差异无统计学意义(P>0.05);MMP-9、C-erbB2 mRNA阳性表达率在有无淋巴结转移患者间比较差异有统计学意义(P<0.05),而hMAM mRNA阳性表达率在有无淋巴结转移患者间比较差异无统计学意义(P>0.05);三者在不同患者年龄、肿瘤大小、病理类型、ER和PR患者间比较差异无统计学意义(P>0.05);其中在HER-2阳性患者中MMP-9 mRNA阳性表达率较HER-2阴性患者高,但差异无统计学意义(P>0.05),而hMAM mRNA阳性表达率比较差异有统计学意义(P<0.05)。见表 2。
| [n(η/%)] | |||||||
| Characteristic | n | MMP-9 mRNA | P | C-erbB2 mRNA | P | hMAM mRNA | P |
| Age(year) | 0.898 | 0.792 | 0.825 | ||||
| ≤ 50 | 42 | 23 (54.76) | 13 (30.95) | 16 (38.10) | |||
| >50 | 32 | 18 (56.25) | 9 (28.13) | 13 (40.63) | |||
| Tumor size(d/cm) | 0.632 | 0.723 | 0.093 | ||||
| ≤2 | 28 | 13 (46.43) | 11 (39.29) | 9 (32.14) | |||
| >2 | 46 | 24 (52.17) | 20 (43.48) | 24 (52.17) | |||
| TNM stage | 0.579 | 0.085 | 0.085 | ||||
| Ⅰ | 11 | 4 (36.36) | 3 (27.27) | 2 (18.18) | |||
| Ⅱ | 17 | 9 (52.94) | 6 (35.29) | 4 (23.53) | |||
| Ⅲ | 29 | 1 (58.62) | 15 (51.72) | 12 (41.38) | |||
| Ⅳ | 17 | 12 (70.59) | 12 (70.59) | 10 (58.82) | |||
| LM | 0.011 | 0.018 | 0.150 | ||||
| Yes | 39 | 26 (66.67) | 23 (58.97) | 21 (53.85) | |||
| No | 35 | 13 (37.14) | 11 (31.43) | 13 (37.14) | |||
| ER | 0.703 | 0.935 | 0.941 | ||||
| Negtive | 55 | 32 (58.18) | 15 (27.27) | 14 (25.45) | |||
| Positive | 19 | 12 (63.16) | 5 (26.32) | 5 (26.32) | |||
| PR | 0.966 | 0.695 | 0.817 | ||||
| Negtive | 53 | 30 (56.60) | 15 (28.30) | 14 (26.42) | |||
| Positive | 21 | 12 (57.14) | 5 (23.81) | 5 (23.81) | |||
| HER-2 | 0.883 | 0.000 | 0.016 | ||||
| Negtive | 56 | 30 (53.57) | 0 (0.00) | 14 (25.00) | |||
| Positive | 18 | 10 (55.56) | 16 (88.89) | 10 (55.56) | |||
| Pathological type | 0.732 | 0.833 | 0.551 | ||||
| IDC | 61 | 36 (59.02) | 17 (27.87) | 14 (22.95) | |||
| ILC | 13 | 7 (53.85) | 4 (30.77) | 4 (30.77) | |||
| LM: Lymphatic metastasis; ER: Restrogen receptor; PR: Progesterone receptor; IDC: Infitrating ductal carcinoma; ILC: Infiltrating lobular carcinoma. | |||||||
hMAM mRNA在健康人中未检测到,乳腺纤维腺瘤组仅检测到1例阳性表达。与健康对照组和乳腺纤维腺瘤组比较,乳腺癌组患者hMAM mRNA不论是单独还是联合表达阳性率均明显增加;乳腺癌组患者hMAM mRNA阳性表达率与健康对照组和乳腺纤维腺瘤组比较均增加(P<0.05),hMAM与MMP-9联合阳性表达率从37.84%增加到59.46%,与乳腺纤维腺瘤组比较差异有统计学意义(P<0.05);与C-erbB2联合检测阳性表达率从37.84%增加到48.65%,与健康对照组和乳腺纤维腺瘤组比较差异均有统计学意义(P<0.05);而在乳腺癌患者中3种基因联合阳性表达率更高,达到64.86%,与乳腺纤维腺瘤组比较差异有统计学意义(P<0.05)。与非乳腺癌组(健康对照组+乳腺纤维腺瘤组)比较,乳腺癌组患者hMAM mRNA单独和联合表达阳性率差异均有统计学意义(P<0.05)。见表 3。
| [n(η/%)] | |||||
| Group | n | hMAM | MMP-9 +hMAM | C-erbB2+hMAM | MMP-9 + C-erbB2+ hMAM |
| Healthy control | 10 | 0 (0.00)*a | 3 (30.00) | 1 (10.00)*b | 4 (40.00) |
| Breast fibroadenoma | 21 | 1 (4.76)**c | 7 (33.33)*d | 3 (14.29)**e | 8 (38.10)*f |
| Breast cancer | 74 | 28 (37.84)△A | 44 (59.46)△△B | 36 (48.65)△△C | 48 (64.86)△D |
| aχ2 =5.676, bχ2= 5.339, cχ2 = 8.438, dχ2 = 4.491, eχ2 = 7.982, fχ2 = 4.844, *P<0.05, ** P<0.01 compared with breast cancer group; Aχ2 = 13.093, Bχ2 = 6.471, Cχ2= 11.837, Dχ2 = 6.103, △P<0.05, △△P<0.01 compared with healthy control group+ breast fibroadenoma group. | |||||
随着肿瘤临床分期的增加,hMAM mRNA单独和联合阳性表达率均逐渐增加,与Ⅰ+Ⅱ期比较,Ⅲ+Ⅳ期乳腺癌患者hMAM及联合C-erbB2 mRNA阳性表达率均增加(P<0.05)。见表 4。
| [n(η/%)] | |||||
| TNM stage | n | hMAM | hMAM+MMP-9 | hMAM+C-erbB2 | hMAM+MMP-9+C-erbB2 |
| Ⅰ+Ⅱ | 28 | 6 (21.43) | 14 (50.00) | 9 (32.14) | 16 (57.14) |
| Ⅲ+Ⅳ | 46 | 22 (47.83) *a | 30 (65.22) | 27 (58.70)*b | 32 (69.57) |
| aχ2 = 5.157, bχ2 = 4.912, *P<0.05 compared withstageⅠ+ Ⅱ. | |||||
hMAM在血液和骨髓中不表达,在正常乳腺上皮组织表达水平较低,而在乳腺癌组织中高表达,其他肿瘤,如直肠癌、胃癌、卵巢癌和前列腺癌等均不表达[9-12],与CK-19、MUC-1和CEA等肿瘤标志物相比具有乳腺组织高特异性[13-14]。有研究[15]表明:hMAM与乳腺癌细胞的侵袭和转移有关。MMPs是一种促进肿瘤血管生成和降解细胞外基质最重要的酶类,而MMP-9是MMPs中相对分子质量最大的酶,主要通过降解细胞外基质和基底膜的结构蛋白Ⅳ型胶原,使肿瘤细胞具有侵袭能力,可在恶性肿瘤中高表达[16],与乳腺癌转移和浸润有关[17-18]。C-erbB2在25%乳腺癌患者中存在过表达或基因异常扩增,控制着乳腺癌细胞的生长,在乳腺癌患者中,C-erbB2 mRNA过表达,与侵袭性表型和不良预后相关[19]。
本研究采用实时荧光定量PCR法对乳腺癌患者外周血中hMAM联合MMP-9及C-erbB2 mRNA进行检测,结果显示:hMAM mRNA阳性表达率与HER-2表达有关,与ER、PR表达及淋巴结是否无关。吴娜萍等[20]发现:hMAM mRNA阳性表达率与HER-2、ER和PR表达及淋巴结转移均无关;鲍慧铮等[7]也发现:hMAM mRNA表达阳性率与淋巴转移无关;颜蕴文等[21]发现:hMAM mRNA阳性表达与ER、HER-2表达和淋巴结是否转移有关,与PR表达无关;不同研究中hMAM mRNA阳性表达率与乳腺癌临床病理特征关系不尽相同,可能是因样本量和纳入标准存在差异而产生,应在大样本大数据前提下进行统计分析进一步证实。葛明广[22]通过实时荧光定量PCR法检测发现:外周血hMAM mRNA阳性表达率为32.1%;鲍慧铮等[7]通过实时荧光定量PCR法检测发现:外周血hMAM mRNA阳性表达率为38.6%;有文献[23-24]报道:常规逆转录PCR检测外周血hMAM mRNA阳性表达率为12%和18%,体现了实时荧光定量PCR的高敏感性特点。本研究结果显示:乳腺癌患者外周血hMAM mRNA阳性表达率[37.84%(28/74)]明显高于健康人和乳腺纤维腺瘤患者,与以往报道相符;与MMP-9及C-erbB2 mRNA联合表达,其阳性表达率也明显高于健康人及乳腺纤维腺瘤患者。本研究还发现:hMAM mRNA与C-erbB2 mRNA联合检测,乳腺癌患者与健康人及乳腺纤维腺瘤患者阳性表达率比较差异均具有统计学意义;而与MMP-9联合检测,在乳腺纤维腺瘤患者阳性表达率差异有统计学意义,在健康人中阳性表达率差异无统计学意义,这可能与本研究健康对照组入组只有10名,样本量太少有关。研究表明:随着分期增加,hMAM mRNA阳性表达率增加[25],与Ⅰ+Ⅱ期比较,Ⅲ+Ⅳ期乳腺癌患者hMAM及联合C-erbB2 mRNA阳性表达率增加,与文献报道一致[26]。
综上所述,本研究结果显示:实时荧光定量PCR法检测乳腺癌外周血hMAM具有一定阳性率,而联合MMP-9及C-erbB2检测可以提高检测阳性率,应用于临床诊断乳腺癌微转移具有一定价值,hMAM检测的标准化问题有待进一步研究。
| [1] | Watson MA, Fleming TP. Mammaglobin, a mammary specific member of the uteroglobin gene family is overexpressed in human breast cancer[J]. Cancer Res, 1996, 56(4): 860–865. |
| [2] | Becker RM, Darrow C, Zimonjie DB, et al. Identification of mammaglobin B, a novel member of the uteroglobin gene family[J]. Genomies, 1998, 54(1): 70–78. DOI:10.1006/geno.1998.5539 |
| [3] | Li C, Zhang T. Human mammaglobin:A specific marker for breast cancer prognosis[J]. J BUON, 2016, 21(1): 35–41. |
| [4] | 陈月凤, 杨华伟, 莫钦国, 等. CK19和hMAM及SBEM检测乳腺癌微转移价值分析[J]. 中华肿瘤防治杂志, 2014(3): 198–202. |
| [5] | Bozhenko VK, Kharchenko NV, Vaskevich EF, et al. Mammaglobin in peripheral blood and tumor in breast cancer patients[J]. Biomed Khim, 2016, 62(4): 453–457. DOI:10.18097/pbmc20166204453 |
| [6] | Bölke E, Orth K, Gerber PA, et al. Gene expression of circulating tumour cells in breast cancer patients[J]. Eur J Med Res, 2009, 14(10): 426–432. |
| [7] | 鲍慧铮, 陈力, 李思莹, 等. CKl9联合hMAM检测乳腺癌循环肿瘤细胞的临床价值[J]. 肿瘤, 2014(2): 153–157. DOI:10.3781/j.issn.1000-7431.2014.02.009 |
| [8] | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) method[J]. Methods, 2001, 25(4): 402–408. DOI:10.1006/meth.2001.1262 |
| [9] | 杨超, 康炜, 李建华, 等. 血清乳腺珠蛋白与乳腺癌的相关性及临床意义探讨[J]. 检验医学, 2014, 29(1): 61–64. |
| [10] | 印滇, 王亚非, 杨莉, 等. hMAM水平与乳腺癌早期诊断和癌微转移的相关性及临床意义研究[J]. 临床和实验医学杂志, 2015, 14(18): 1512–1515. DOI:10.3969/j.issn.1671-4695.2015.18.010 |
| [11] | GoedegebuurePS, Watson MA, Carsten T, et al. Mammaglobin-based strategies for treatment of breast cancer[J]. Curr Cancer Drug Targets, 2017, 4(6): 531–542. |
| [12] | Al Joudi FS. Human mammaglobin in breast cancer:a brief review of its clinical utility[J]. Indian J Med Res, 2014, 139(5): 675–685. |
| [13] | Marchetti A, Buttitta F, Bertacca G, et al. mRNA markers of breast cancer nodal metastases:comparision between mammaglobin and carcinoembryonic antigen in 248 patients[J]. J Pathol, 2001, 195(2): 186–190. DOI:10.1002/(ISSN)1096-9896 |
| [14] | Chen Y, Zou TN, Wu ZP, et al. Detection of cytokeratin 19, human mammaglobin, and carcinoembryonic antigen-positive circulating tumor cells by three-marker reverse transcription-PCR assay and its relation to clinical outcome in early breast cancer[J]. Int J Biol Markers, 2010, 25(2): 59–68. |
| [15] | Koh EH, Cho YW, Mun YJ, et al. Upregulation of human mammaglobin reduces migration and invasion of breast cancer cells[J]. Cancer Invest, 2014, 32(1): 22–29. DOI:10.3109/07357907.2013.861473 |
| [16] | Candido S, Abrams SL, Steelman LS, et al. Roles of NGAL and MMP-9 in the tumor microenvironment and sensitivity to targeted therapy[J]. Biochim Biophys Acta, 2016, 1863(3): 438–448. DOI:10.1016/j.bbamcr.2015.08.010 |
| [17] | 张弘, 姜义, 关文曾, 等. 乳腺癌组织中基质金属蛋白酶2、9活性的测定[J]. 吉林大学学报:医学版, 2002, 28(6): 591–599. |
| [18] | 孙亚楠, 毛晓韵, 刘晓虹, 等. 基底细胞样乳腺癌患者乳腺癌和癌旁正常乳腺组织中MTDH与MMP-9蛋白表达的相关性[J]. 吉林大学学报:医学版, 2016, 42(3): 530–534, 639. |
| [19] | Browne BC, O'Brien N, Duffy MJ, et al. HER-2 signaling and inhibition in breast cancer[J]. Curr Cancer Drug Targets, 2009, 9(3): 419–438. DOI:10.2174/156800909788166484 |
| [20] | 吴娜萍, 肇毅, 王珏, 等. 联合多标志物检测乳腺癌外周血循环肿瘤细胞与分子分型的相关性及临床意义[J]. 中华实验外科杂志, 2014(5): 935–937. |
| [21] | 颜蕴文, 张敬杰, 徐晓军, 等. 联合检测乳腺癌患者外周血中细胞角蛋白19及人乳腺球蛋白mRNA的意义[J]. 中华乳腺病杂志:电子版, 2010(2): 45. |
| [22] | 葛明广. 荧光定量PCR法检测人乳腺珠蛋白与乳腺癌微小转移诊断的研究[J]. 中外医疗, 2008(28): 25–27. DOI:10.3969/j.issn.1674-0742.2008.28.014 |
| [23] | Roncella S, Ferro P, Bacigalupo B, et al. Human mammaglobin mRNA is a reliable molecular marker for detecting occult breast cancer cells in peripheral blood[J]. J Exp Clin Cancer Res, 2005, 24(2): 265–271. |
| [24] | Lin YC, Wu Chou YH, Liao IC, et al. The expression of mammaglobin mRNA in peripheral blood of metastatic breast cancer patients as an adjunct to serum tumor markers[J]. Cancer Lett, 2003, 191(1): 93–99. DOI:10.1016/S0304-3835(02)00545-1 |
| [25] | Liu Y, Ma L, Liu X, et al. Expression of human mammaglobin as a marker of bone marrow micrometastasis in breast cancer[J]. Exp Ther Med, 2012, 3(3): 550–554. DOI:10.3892/etm.2011.429 |
| [26] | Lee GW, Kim JY, Koh EH, et al. Plasma human mammaglobin mRNA associated with poor outcome in patients with breast cancer[J]. Genet Mol Res, 2012, 11(4): 4034–4042. DOI:10.4238/2012.November.28.2 |
 2017, Vol. 43
2017, Vol. 43


