扩展功能
文章信息
- 文思敏, 于多, 吕欣, 王铁君
- WEN Simin, YU Duo, LYU Xin, WANG Tiejun
- 紫杉醇联合顺铂与单药顺铂2种同步放化疗方案对中晚期宫颈癌患者预后的影响及安全性评价
- Influence of docetaxel combined with cisplatinand single-agent cisplatin concurrent chemoradiotherapy in prognosis of patients with advanced cervical cancer and evaluation on security
- 吉林大学学报(医学版), 2017, 43(05): 1002-1008
- Journal of Jilin University (Medicine Edition), 2017, 43(05): 1002-1008
- 10.13481/j.1671-587x.20170527
-
文章历史
- 收稿日期: 2017-03-06
2. 吉林大学第二医院放疗科, 吉林 长春 130041;
3. 吉林大学公共卫生学院流行病与卫生统计学教研室, 吉林 长春 130021
2. Department of Radiotherapy, Second Hospital, Jilin University, Changchun 130041, China;
3. Department of Epidemiology and Health Statistics, School of Public Health, Jilin University, Changchun 130021, China
宫颈癌作为一种严重威胁女性生命健康的疾病,其治疗意义不容忽视。在过去的十几年中,同步放化疗的开展使宫颈癌患者的生存率明显上升[1]。针对同步放化疗方案的选择,合理选择药物及制订给药方案是影响疗效、降低毒性的重要因素。近年来国内外一些研究和大型临床随机对照试验[2-6]发现:紫杉醇在早期以及晚期的宫颈癌患者治疗中,单独使用或者与铂类联合使用均具有较强的功效,在早期宫颈癌的同步放化疗中,紫杉醇联合顺铂(docetaxel combined with cisplatin, TP)方案明显优于单药顺铂且毒性是可耐受的。一项二期临床随机对照试验[4]显示:在Ⅳ期及复发的宫颈癌患者中,紫杉醇联合顺铂用药的无进展生存期(progression-free survival, PFS)更长。紫杉醇联合顺铂方案在宫颈癌同步放化疗的应用中有着重要地位。在临床研究中,对同步放化疗最佳方案说法不同,如何将顺铂合理地应用于宫颈癌同步放化疗中是亟待解决的问题。尚未有关于行紫杉醇联合顺铂与单药顺铂根治性同步放化疗的中晚期宫颈癌患者的预后及安全性评价的相关研究。本研究对2010年1月—2012年1月在吉林大学第二医院行根治性同步放化疗的中晚期宫颈癌患者进行生存分析,分析单药顺铂和TP方案对中晚期宫颈癌综合治疗的安全性及对患者预后的影响,旨在改善局部中晚期宫颈癌患者的生存情况,探讨采用紫杉醇联合顺铂同步放化疗治疗中晚期宫颈癌的潜在应用价值。
1 资料与方法 1.1 研究对象通过吉林大学第二医院病案查询系统选取2010年1月—2012年1月在本院行根治性同步放化疗的中晚期宫颈癌患者。纳入标准:经病理学检查明确诊断为宫颈癌;患者年龄在18~75岁;卡氏(Karnofsky)评分70以上;美国东部肿瘤协作组(Eastern Cooperative Oncology Group,ECOG)评分0~1分;根据宫颈癌国际妇产科联盟(international federation of gynecology and obstetrics,FIGO)标准选定临床分期为ⅡB~ⅣB;所有患者均行体外照射(external beam radiation therapy,EBRT)联合高剂量率腔内近距离治疗(high-dose rate intracavitary brachytherapy,HDR-ICBT),在体外照射治疗剂量达DT:40 Gy后给予腔内照射治疗;外照射PTV总剂量为DT:40~52 Gy, 1.8~2.0 Gy/f。腔内近距离治疗单次剂量6~7 Gy;选择化疗方案为TP方案(紫杉醇135 mg·m-2,d1;顺铂70 mg·m-2,d1,周期21 d)或单药顺铂方案(顺铂50 mg,d1,周期7 d)进行同步放化疗的患者。排除标准:术后及盆腔放疗史的患者。本组共纳入218例研究对象。对选取的218例患者根据其同步放化疗方案分为单药顺铂组(113例)和紫杉醇联合顺铂组(105例)。2组患者临床资料比较差异无统计学意义(P>0.05),具有可比性。见表 1。
| [n(η/%)] | ||||||||||||||||||||
| Group | n | Age(year) | Tumor diameter(d/cm) | Clinical stage | Pathological grade | Pathological type | Primiparous age(year) | |||||||||||||
| ≤ 50 | >50 | ≤4 | >4 | ⅡB | Ⅲ | Ⅳ | Low | High | Squamous carcinoma Adenocarcinoma | Other | ≤ 20 | >20 | ||||||||
| Single-agent cisplatin | 113 | 63(55.8) | 50(44.2) | 16(14.2) | 97(85.8) | 48(42.5) | 58(51.3) | 7(6.2) | 34(30.1) | 79(69.9) | 71(62.8) | 30(26.5) | 12(10.7) | 15(13.6) | 95(86.4) | |||||
| Docetaxel combined with cisplatin | 105 | 54(51.4) | 51(48.6) | 19(18.1) | 86(81.9) | 37(35.2) | 65(61.9) | 3(2.9) | 35(33.3) | 70(66.7) | 70(66.7) | 22(21.0) | 13(12.3) | 13(12.9) | 88(87.1) | |||||
利用病案查询系统回顾性收集218例患者的基本资料,包括就诊年龄、肿瘤直径、临床分期、化疗方案、病理等级、病理类型、初产年龄和治疗后出现的不良反应情况。不良反应情况根据美国国家癌症研究所关于不良事件的通用术语标准(National Cancer Institute Common Terminology Criteria for Adverse Events, CTCAE)3.0标准分级,分为0~4级。本研究观察的不良反应主要包括白细胞减少症、嗜中性粒细胞减少症、血小板减少症、贫血、疲乏、恶心、腹痛、呕吐、腹泻、泌尿生殖系损伤、谷草转氨酶、谷丙转氨酶、肌酐和厌食。
1.3 随访随访信息来源于电话随访及门诊、住院复查资料。随访时间自患者接受治疗之日起,截止至2017年1月。失访主要发生于患者治疗后的3~5年,其中治疗后3年失访者7例,治疗后4年失访者11例,至2017年1月31日末次随访,共失访17例,失访率为7.8%。中位随访时间为65.21个月。死亡79例,截尾数据(截止,失访)139例。患者的生存时间为患者自接受治疗之日起直至患者死亡的时期,对于失访和健在的患者,其生存期为治疗日至最后一次随访日期。
1.4 统计学分析采用SPSS 18.0统计软件进行统计学分析。2组中晚期宫颈癌患者的临床病理特征等计数资料组间比较采用应用χ2检验或Fisher精确概率法。将患者的各项不良反应分为0~4级,采用非参数检验(Mann-Whitney U检验)的方法比较2组患者的各项不良反应发生情况。采用Kaplan-Meier法绘制生存曲线,Log-rank检验生存率,影响患者预后的单因素及多因素分析采用Cox比例风险回归模型。以P<0.05为差异有统计学意义。
2 结果 2.1 2组中晚期宫颈癌患者的临床病理特征2组患者的年龄、肿瘤直径、临床分期、病理等级、病理类型和初产年龄比较差异无统计学意义(P>0.05)。约有半数患者年龄在50岁以上,大多数患者的肿瘤直径大于4 cm,临床分期处于ⅡB期和Ⅲ期,病理等级处于高等级(即低分化水平),鳞癌病理类型居多。除未生过子女的7例患者(单药顺铂组3例,紫杉醇联合顺铂组4例)外,大多数患者的初产年龄在20岁以上。见表 1。
2.2 行不同化疗方案的中晚期宫颈癌患者的生存率218例患者的1、3和5年生存率分别为96.33%、76.15%和63.76%,紫杉醇联合顺铂组患者的1、3和5年生存率分别为97.14%、80.0%和73.33%,单药顺铂组方案患者的1、3和5年生存率分别为95.58%、72.57%和54.87%,紫杉醇联合顺铂组患者5年生存率高于单药顺铂组(χ2=8.032,P=0.005)。见表 2。
| (η/%) | ||||
| Group | n | 1-year OS rate | 3-year OS rate | 5-year OS rate |
| Single-agent cisplatin | 113 | 95.58 | 72.57 | 54.87 |
| Docetaxel combined with cisplatin | 105 | 97.14 | 80.00 | 73.33* |
| * P<0.01 compared with single-agent cisplatin. | ||||
单因素分析:与预后有关的因素是患者的肿瘤直径、临床分期、化疗方案和病理类型为腺癌; 而患者年龄、病理等级、其他病理类型及初产年龄与预后无关。将单因素分析结果中具有统计学意义的变量纳入到多因素分析中,结果显示:患者的临床分期; Ⅲ期[P=0.016, HR(95%CI)= 1.90(1.13~3.19);Ⅳ期:P<0.001, HR(95%CI)= 19.13(7.84~46.68)]、化疗方案[P=0.009, HR(95%CI)= 0.54(0.34~0.86)]和病理类型为腺癌[P=0.021, HR(95%CI)= 1.88(1.10~3.21)]是其预后的独立影响因素,且临床分期处于ⅡB期、采用紫杉醇联合顺铂同步放化疗方案和病理类型为鳞癌的患者预后较好。见表 3。
| Characteristic | Univariate analysis | Multivariate analysis | |||
| HR(95%CI) | P | HR(95%CI) | P | ||
| Age(year) | |||||
| ≤ 50 >50 | Reference 1.21(0.78-1.89) | 0.391 | |||
| Tumor diameter(cm) | |||||
| ≤4 >4 | Reference 2.29(1.05-4.98) | 0.037 | Reference 1.87(0.85-4.09) | 0.120 | |
| Clinical stage | |||||
| ⅡB | Reference | Reference | |||
| Ⅲ | 1.76(1.05-2.94) | 0.031 | 1.90(1.13-3.19) | 0.016 | |
| Ⅳ | 30.36(13.20-69.83) | <0.001 | 19.13(7.84-46.68) | <0.001 | |
| Chemotherapy treatment | |||||
| Single-agent cisplatin | Reference | Reference | |||
| Docetaxel combined with cisplatin | 0.54(0.34-0.86) | 0.010 | 0.54(0.34-0.86) | 0.009 | |
| Pathological grade | |||||
| Low High | Reference 1.59(0.95-2.66) | 0.080 | |||
| Pathological type | |||||
| Squamous carcinoma | Reference | Reference | |||
| Adenocarcinoma | 2.48(1.53-4.02) | <0.001 | 1.88(1.10-3.21) | 0.021 | |
| Other | 1.46(0.73-2.92) | 0.280 | 1.43(0.71-2.85) | 0.316 | |
| Primiparous age(year) | |||||
| ≤ 20 >20 | Reference 0.82 (0.44-1.51) | 0.519 | |||
图 1为Kaplan-Meier法绘制多因素分析具有统计学意义的不同临床分期、化疗方案及病理类型的中晚期宫颈癌患者的总体生存曲线的比较。其中,与临床分期为Ⅲ期及Ⅳ期患者比较,ⅡB期患者的预后较好,而Ⅳ期患者预后最差(图 1A);紫杉醇联合顺铂组患者的预后较单药顺铂组患者预后好,生存曲线较高(图 1B)。不同病理类型患者的总体生存曲线的比较,其中病理类型为鳞癌的患者预后最好,而病理类型为腺癌的患者预后最差,其他病理类型患者的生存曲线居中,较鳞癌患者预后差,但差异无统计学意义(P>0.05)(图 1C)。
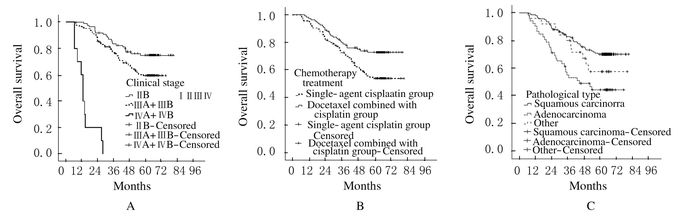
|
| 图 1 Kaplan-Meier法绘制不同临床分期(A)、化疗方案(B)和病理类型(C)中晚期宫颈癌患者的总体生存曲线 Figure 1 Overall survival curves of advanced cervical cancer patients with different clinical stages (A), chemotherapy treatments (B) and pathological types (C) drawn with Kaplan-Meier method |
|
|
大部分毒性反应集中在0~2级,3级以上不良反应所占比例较少。与紫杉醇联合顺铂组患者比较,单药顺铂组患者白细胞减少症(Z=-2.060,P=0.039) 和嗜中性粒细胞减少症(Z=-2.246,P=0.025)2项不良反应较轻,差异有统计学意义。2组患者的血小板减少症、贫血、疲乏、恶心、腹痛、呕吐、腹泻、泌尿生殖系损伤、谷草转氨酶、谷丙转氨酶、肌酐和厌食12项不良反应比较差异均无统计学意义(P>0.05)。见表 4。
| Group | n | Leucopenia | Neutropenia | Thrombopenia | Anemia | Fatigue | Nausea | Abdominal pain | |
| Single-agent cisplatin | 113 | ||||||||
| 0 | 63(56.8) | 66(60.6) | 75(68.8) | 70(64.2) | 83(76.1) | 26(23.9) | 66(60.6) | ||
| 1 | 35(31.5) | 30(27.5) | 21(19.3) | 19(17.4) | 10(9.2) | 29(26.6) | 33(30.3) | ||
| 2 | 12(10.8) | 10(9.2) | 7(6.4) | 15(13.8) | 10(9.2) | 35(32.1) | 8(7.3) | ||
| 3 | 1(0.9) | 3(2.7) | 6(5.5) | 3(2.8) | 6(5.5) | 15(13.7) | 2(1.8) | ||
| 4 | 0(0.0) | 0(0.0) | 0(0.0) | 2(1.8) | 0(0.0) | 4(3.7) | 0(0.0) | ||
| Mean rank | 100.33 | 97.75 | 99.53 | 107.66 | 102.92 | 109.32 | 107.99 | ||
| Docetaxel combined with cisplatin | 105 | ||||||||
| 0 | 54(51.9) | 48(47.1) | 59(57.8) | 68(66.7) | 71(70.2) | 27(26.5) | 68(67.3) | ||
| 1 | 16(15.4) | 29(28.4) | 19(18.7) | 18(17.6) | 15(14.9) | 30(29.4) | 20(19.8) | ||
| 2 | 19(18.3) | 25(24.5) | 18(17.6) | 14(13.7) | 10(9.9) | 31(30.4) | 13(12.9) | ||
| 3 | 13(12.5) | 0(0.0) | 6(5.9) | 0(0.0) | 2(2.0) | 12(11.7) | 0(0.0) | ||
| 4 | 2(1.9) | 0(0.0) | 0(0.0) | 2(2.0) | 3(3.0) | 2(2.0) | 0(0.0) | ||
| Mean rank | 116.19 | 114.81 | 112.91 | 104.23 | 108.28 | 102.46 | 102.81 | ||
| Group | n | Emesis | Diarrhea | Genitourinary injuries | AST | ALT | reatinine | Anorexia | |
| Single-agent cisplatin | 113 | ||||||||
| 0 | 38(34.9) | 58(53.2) | 89(81.7) | 101(91.0) | 97(87.4) | 106(95.5) | 56(50.5) | ||
| 1 | 36(33.0) | 35(32.1) | 18(16.5) | 9(8.1) | 35(31.5) | 30(27.5) | 21(19.3) | ||
| 2 | 25(22.9) | 14(12.8) | 0(0.0) | 1(0.9) | 3(2.7) | 0(0.0) | 24(21.6) | ||
| 3 | 10(9.2) | 2(1.9) | 2(1.8) | 0(0.0) | 0(0.0) | 0(0.0) | 3(2.7) | ||
| 4 | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | ||
| Mean rank | 98.57 | 110.38 | 106.77 | 105.64 | 109.39 | 103.25 | 99.42 | ||
| Docetaxel combined with cisplatin | 105 | ||||||||
| 0 | 26(25.5) | 65(66.4) | 87(87.0) | 91(91.9) | 94(94.0) | 90(90.0) | 37(36.6) | ||
| 1 | 33(32.4) | 22(22.4) | 1(1.0) | 0(0.0) | 6(6.0) | 10(10.0) | 35(34.7) | ||
| 2 | 26(25.4) | 11(11.2) | 9(9.0) | 8(8.1) | 0(0.0) | 0(0.0) | 20(19.8) | ||
| 3 | 15(14.7) | 0(0.0) | 2(2.0) | 0(0.0) | 0(0.0) | 0(0.0) | 9(8.9) | ||
| 4 | 2(2.0) | 0(0.0) | 1(1.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | ||
| Mean rank | 113.94 | 96.91 | 103.08 | 105.35 | 102.24 | 109.05 | 114.28 | ||
| AST:Aspartate transaminase; ALT: Alanine transaminase. | |||||||||
本研究中,单因素分析及多因素分析均显示临床分期是影响中晚期宫颈癌预后的因素,且处于Ⅲ期及Ⅳ期的患者较ⅡB期的患者预后较差。目前国内外已有大量文献证实临床分期是影响宫颈癌患者预后的重要因素。
不同化疗方案对同步放化疗患者的预后有着不可忽视的影响。Takatori等[7]研究显示:应用顺铂与紫杉醇联合用药化疗患者的无进展生存期要长于顺铂单药化疗患者,与此同时,分别接受紫杉醇和顺铂联用的化疗方案与单独使用顺铂作为化疗方案的患者的总客观反应率(36%和19%)和完全缓解率(15%和6%)呈现明显的优势。Kim等[5]发现:在早期宫颈癌患者中,针对手术切缘呈阳性等高风险因素早期宫颈癌患者(ⅡA和ⅢB)中,行联合方案进行同步放化疗能够明显提高肿瘤局控率。在本研究中,单因素分析及多因素分析均显示:紫杉醇联合顺铂作为同步放化疗方案与单药顺铂比较预后较好。对于中晚期宫颈癌患者,为了延长其生存时间,是否将紫杉醇与顺铂联合用药的同步放化疗方案广泛应用于临床,仍需随机化大样本的研究进一步验证。
病理类型是影响中晚期宫颈癌患者预后的重要因素,在本研究中,腺癌病理类型是影响预后的独立危险因素,鳞癌患者的预后要明显优于腺癌患者。Huang等[8]研究证实:对于存在深部基质浸润、阳性切缘或淋巴结转移等高危因素的患者,行根治性子宫切除术的早期宫颈癌患者(ⅠB~ ⅡB),其术后采取辅助放射治疗或者同步放化疗,病理类型为腺癌和鳞腺癌的生存率明显低于病理类型为鳞癌的患者。文献[9-11]报道:对于接受单纯放射性治疗的患者,腺癌及鳞腺癌的生存率较鳞癌亚型差。这与本研究结果相似。因此,病理类型的不同可能导致接受同步放化疗患者的预后不同,并且病理类型为腺癌的患者在接受根治性放射治疗时,具有更高的风险。这些可进一步解释宫颈腺癌患者存在潜在的辐射抗性。
在对化疗方案进行评估的过程中,化疗方案的有效性和安全性均应作为评价标准。研究[12]表明:在宫颈癌放化疗治疗方案的评价中,直接细胞毒性是不可忽视的评价标准。然而当放射治疗与同步放化疗共同作用时,可能会增加患者的不良反应,包括腹泻和便血等。研究[13]表明:运用先进的外照射技术会大幅度降低胃肠道和泌尿生殖系的不良反应。这在很大程度上对宫颈癌同步放化疗的患者起到了保护作用。但当放射治疗与化疗联合时,会导致毒性反应增强。一项前瞻性研究[14]显示:吉西他滨联合顺铂在晚期宫颈癌同步放化疗的应用中,3级嗜中性粒细胞减少症和3级血小板减少症发生率分别为45.0%和5.4%。另有三期临床随机对照试验[15-17]显示:拓扑替康联合顺铂在宫颈癌切除术后同步放化疗中,血液毒性极强,乃至试验提前关闭。本研究中单药顺铂组患者与顺铂联合紫杉醇组患者比较,仅有白细胞减少症及嗜中性粒细胞减少症2项不良反应较轻,而2组间其他不良反应比较差异则无统计学意义,可以认为该2种化疗方案的不良反应程度相当。与单药顺铂组比较,紫杉醇联合顺铂组患者的预后较好,且不良反应与单药顺铂组比较无明显差异。本研究为宫颈癌同步放化疗中化疗方案的制定提供了一定的参考,针对临床分期较晚及腺癌病理类型的宫颈癌患者,可适当调整治疗方案采取个性化治疗。
| [1] | Pereira E, Cooper HH, Zelaya PG, et al. Concurrent chemoradiation versus radiotherapy alone for the treatment of locally advanced cervical cancer in a low-resource setting[J]. Gynecol Oncol Rep, 2017, 19: 50–52. DOI:10.1016/j.gore.2016.12.006 |
| [2] | Alberts DS, Blessing JA, Landrum LM, et al. Phase Ⅱ trial of nab-paclitaxel in the treatment of recurrent or persistent advanced cervix cancer:A gynecologic oncology group study[J]. Gynecol Oncol, 2012, 127(3): 451–455. DOI:10.1016/j.ygyno.2012.09.008 |
| [3] | Monk BJ, Sill MW, McMeekin DS, et al. Phase Ⅲ trial of four cisplatin-containing doublet combinations in stage ⅣB, recurrent, or persistent cervical carcinoma:a Gynecologic Oncology Group study[J]. J Clin Oncol, 2009, 27(28): 4649–4655. DOI:10.1200/JCO.2009.21.8909 |
| [4] | Walker JL, Morrison A, DiSilvestro P, et al. A phase Ⅰ/Ⅱ study of extended field radiation therapy with concomitant paclitaxel and cisplatin chemotherapy in patients with cervical carcinoma metastatic to the para-aortic lymph nodes:a Gynecologic Oncology Group study[J]. Gynecol Oncol, 2009, 112(1): 78–84. DOI:10.1016/j.ygyno.2008.09.035 |
| [5] | Kim K, Chie EK, Wu HG, et al. Efficacy of paclitaxel and carboplatin as a regimen for postoperative concurrent chemoradiotherapy of high risk uterine cervix cancer[J]. Gynecol Oncol, 2006, 101(3): 398–402. DOI:10.1016/j.ygyno.2005.10.035 |
| [6] | Wang X, Shen Y, Zhao Y, et al. Adjuvant intensity-modulated radiotherapy (IMRT) with concurrent paclitaxel and cisplatin in cervical cancer patients with high risk factors:A phase Ⅱ trial[J]. Eur J Surg Oncol, 2015, 41(8): 1082–1088. DOI:10.1016/j.ejso.2015.04.018 |
| [7] | Takatori E, Shoji T, Takada A, et al. A retrospective study of neoadjuvant chemotherapy plus radical hysterectomy versus radical hysterectomy alone in patients with stage Ⅱ cervical squamous cell carcinoma presenting as a bulky mass[J]. Onc Targets Ther, 2016, 9: 5651–5657. DOI:10.2147/OTT |
| [8] | Huang YT, Wang CC, Tsai CS, et al. Clinical behaviors and outcomes for adenocarcinoma or adenosquamous carcinoma of cervix treated by radical hysterectomy and adjuvant radiotherapy or chemoradiotherapy[J]. Int J Radiat Oncol Biol Phys, 2012, 84(2): 420–427. DOI:10.1016/j.ijrobp.2011.12.013 |
| [9] | Chen JL, Huang CY, Huang YS, et al. Differential clinical characteristics, treatment response and prognosis of locally advanced adenocarcinoma/adenosquamous carcinoma and squamous cell carcinoma of cervix treated with definitive radiotherapy[J]. Acta Obset Gynecol Scand, 2014, 93(7): 661–668. DOI:10.1111/aogs.2014.93.issue-7 |
| [10] | Huang YT, Wang CC, Tsai CS, et al. Long-term outcome and prognostic factors for adenocarcinoma/adenosquamous carcinoma of cervix after definitive radiotherapy[J]. Int J Radiat Oncol Biol Phys, 2011, 80(2): 429–436. DOI:10.1016/j.ijrobp.2010.02.009 |
| [11] | Rose PG, Java JJ, Whitney CW, et al. Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation[J]. Gynecol Oncol, 2014, 135(2): 208–212. DOI:10.1016/j.ygyno.2014.08.018 |
| [12] | Rose PG. Chemoradiotherapy for cervical cancer[J]. Eur J Cancer, 2002, 38(2): 270–278. DOI:10.1016/S0959-8049(01)00352-5 |
| [13] | Chen MF, Tseng CJ, Tseng CC, et al. Clinical outcome in posthysterectomy cervical cancer patients treated with concurrent Cisplatin and intensity-modulated pelvic radiotherapy:comparison with conventional radiotherapy[J]. Int J Radiat Oncol Biol Phys, 2007, 67(5): 1438–1444. DOI:10.1016/j.ijrobp.2006.11.005 |
| [14] | Duenas-Gonzalez A, Zarba JJ, Patel F, et al. Phase Ⅲ, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage ⅡB to IVA carcinoma of the cervix[J]. J Clin Oncol, 2011, 29(13): 1678–1685. DOI:10.1200/JCO.2009.25.9663 |
| [15] | Sun W, Wang T, Shi F, et al. Randomized phase Ⅲ trial of radiotherapy or chemoradiotherapy with topotecan and cisplatin in intermediate-risk cervical cancer patients after radical hysterectomy[J]. BMC Cancer, 2015, 15: 353. DOI:10.1186/s12885-015-1355-1 |
| [16] | 范淑英, 王健, 刘芸, 等. 紫杉醇联合卡铂周疗和三周疗法治疗卵巢癌的Meta分析[J]. 解放军医学杂志, 2016, 41(7): 589–597. DOI:10.11855/j.issn.0577-7402.2016.07.14 |
| [17] | 李丹明, 王黎, 孙新臣, 等. 中晚期宫颈癌适形调强放疗与容积旋转调强技术临床效果比较[J]. 中国医学物理学杂志, 2016, 33(5): 478–480. |
 2017, Vol. 43
2017, Vol. 43


