扩展功能
文章信息
- 杨冬阳, 赖晓嵘, 黎莹, 马立宇, 罗刚, 李子俊, 徐飞, 马冬
- YANG Dongyang, LAI Xiaorong, LI Ying, MA Liyu, LUO Gang, LI Zijun, XU Fei, MA Dong
- Ⅳ期结直肠癌轻微症状患者原发灶切除和KRAS基因突变对其预后的影响
- Influence of primary resection and KRAS gene mutation in prognosis of mild symptomatic patients with stage Ⅳ colorectal cancer
- 吉林大学学报(医学版), 2017, 43(04): 805-811
- Journal of Jilin University (Medicine Edition), 2017, 43(04): 805-811
- 10.13481/j.1671-587x.20170427
-
文章历史
- 收稿日期: 2017-01-30
2. 广东省人民医院广东省 医学科学院协和医疗中心, 广东 广州 510080;
3. 广东省人民医院广东省医学科学院消化内科, 广东 广州 510080
2. Concord Medical Center, Guangdong General People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China;
3. Department of Gastroenterology, Guangdong General People's Hospital, Guangdong Academy of Medical Sciences, Guangzhou 510080, China
近年来我国结直肠癌的发病率和病死率呈快速上升趋势,其中转移是最常见的相关死亡原因,这是由于Ⅳ期结直肠癌的治疗仍然棘手,治疗及监测手段有限,影响患者预后生存。研究[1-3]显示:Ⅳ期结直肠癌原发灶切除可提高患者生存率,但影响其预后的因素和可能的监测方法尚未明确。KRAS基因突变可以激活下游Raf/Erk/Map激酶和其他信号通路,促进肿瘤细胞的浸润和转移。研究[4]显示:KRAS突变可能影响肿瘤的转移和预后。目前关于Ⅳ期结直肠癌轻微症状患者原发灶切除和KRAS基因突变对预后影响及其与临床病理参数关系的报道较少。本文作者对46例Ⅳ期结直肠癌轻微症状患者行原发灶手术和KRAS基因突变对其预后的影响及其与患者临床病理参数的关系进行分析,并行5年随访,以期为原发灶切除的Ⅳ期结直肠癌症状轻微患者的临床治疗及监测提供依据。
1 资料与方法 1.1 病例选择选择2010年1—12月在广东省人民医院确诊并进行原发灶手术切除的Ⅳ期结直肠癌患者46例,所有患者具有完整病历随访资料,结直肠癌分期标准采用2009年修改的国际抗癌联盟(UICC)和美国肿瘤联合会(AJCC)联合制定的TNM分期法,均为术后病理证实腺癌的Ⅳ期患者。入选标准:病例需存在一项以上轻微症状,包括便频、腹泻或便秘,里急后重、肛门坠胀,腹隐痛、气胀,间歇便血、大便变细;原发灶需行KRAS基因检测;功能状态评分为0~1分。排除标准:伴有腹膜炎、肠梗阻、不可控制的原发灶出血和原发灶穿孔症状;有严重心、肺、肝和肾疾病患者;不能耐受手术的患者。本研究共纳入46例患者,男性28例,女性18例,中位年龄60岁,60岁及以上者23例,小于60岁者23例。按KRAS基因型分为野生型26例和突变型20例;低分化13例,中、高分化33例;肝转移15例,肺转移5例,多发转移17例。研究对象一般情况见表 1。
1.2 标本来源和处理46例Ⅳ期结直肠癌轻微症状患者均行原发灶切除,留取手术切除的肿瘤组织标本,采用直接测序法检测患者的KRAS基因变异情况。
1.3 KRAS基因突变的检测方法和分析在肿瘤原发灶采样取癌组织3 mm×3 mm×5 mm,取材部位避开肿瘤中心坏死灶,标本取材后经固定和石蜡包埋,由广东省人民医院病理科2名病理学专家进行组织学诊断,所有检测均由广东省人民医院医学检验中心完成。① 标本采集及处理:切除的结肠直肠癌新鲜组织标本经固定、脱水透明、浸蜡包埋及切片制成组织切片后,行HE染色。② DNA提取和扩增:KRAS基因突变的检测方法采用目前公认的直接测序法进行。采用Goelz氏DNA提取方法对石蜡包埋组织进行模板DNA提取。引物的设计与合成:参照NCBI基因数据库,根据需要检测的位点设计引物。引物序列:上游为5′-GGCCTGCTGAAAATGACTGA-3′,下游为5′-GTCCTGCACCAGTAATATGC-3′。PCR扩增产物在凝胶成像仪下观察结果,拍照、记录。③ 基因测序:将PCR扩增产物纯化,采用焦磷酸测序技术(Pyrosequencing)进行测序。
1.4 随访对46例患者进行平均5年的跟踪随访,随访患者每3~6个月复查1次,包括胸片、腹部B超和CT等。
1.5 统计学分析采用SPSS21.0统计软件进行统计学分析。Ⅳ期结直肠癌患者原发灶切除和KRAS基因突变与其临床病理参数之间的关系分析采用χ2检验。采用Kaplan-Meier生存曲线评价结直肠癌患者临床病理参数与患者生存期之间的关系, 采用Log-rank检验分析各种因素对Ⅳ期结直肠癌原发灶切除预后的影响。以P < 0.05为差异有统计学意义。
2 结果 2.1 基因分型KRAS为点突变,本组46例结肠直肠癌患者中,KRAS基因突变位于2号外显子的第12和13密码子位点。共20例发生KRAS基因突变,突变率为43.4%,其中12密码子突变和13密码子突变的频率分别为95.7%和4.3%,12和13密码子共突变的频率为0%。
2.2 KRAS基因突变与Ⅳ期结直肠癌患者临床病理参数的关系KRAS基因突变与肿瘤原发部位和肿瘤的多发转移有关联(P < 0.05)。KRAS基因突变与其他临床病理参数如患者性别、年龄、肿瘤分化程度、肝脏转移和肺转移等均无关联(P > 0.05)。见表 1。
| Clinical parameter | n | KRASgene | χ2 | P | |
| Wild type | Mutant type | ||||
| Gender | |||||
| Male | 28 | 15 | 13 | 0.25 | 0.62 |
| Female | 18 | 11 | 7 | ||
| Age (year) | |||||
| ≥60 | 23 | 15 | 8 | 1.42 | 0.23 |
| < 60 | 23 | 11 | 12 | ||
| Tumor site | |||||
| Right colon | 14 | 6 | 8 | 4.49 | 0.034 |
| Left colon | 32 | 20 | 12 | ||
| Differentiation degree | |||||
| Low | 13 | 8 | 5 | 0.19 | 0.67 |
| Medium/high | 33 | 18 | 15 | ||
| Liver metastasis | |||||
| Liver metastasis | 15 | 9 | 6 | 0.11 | 0.74 |
| Other | 31 | 17 | 14 | ||
| Lung metastasis | |||||
| Lung metastasis | 5 | 3 | 2 | 0.02 | 0.91 |
| Other | 41 | 23 | 18 | ||
| Multiplemetastases | |||||
| Multiple metastases | 17 | 7 | 10 | 3.95 | 0.047 |
| Other | 29 | 19 | 10 | ||
截止至2016年1月,仍有16位患者生存,5年生存率为34.8%,总中位生存时间为39.6个月(图 1)。Kaplan-Meier法生存曲线和Log-rank单因素分析显示:KRAS基因野生型患者中位生存时间为58.4个月,KRAS基因突变型患者中位生存时间为42.2个月,组间比较差异无统计学意义(P > 0.05);右半结肠癌患者中位生存时间为34.2个月,左半结肠癌患者中位生存时间为58.3个月,组间比较差异有统计学意义(P < 0.05);肝转移、肺转移和多发转移与结直肠癌患者术后预后不良有密切关系(P < 0.05)。见表 2和图 2~6。
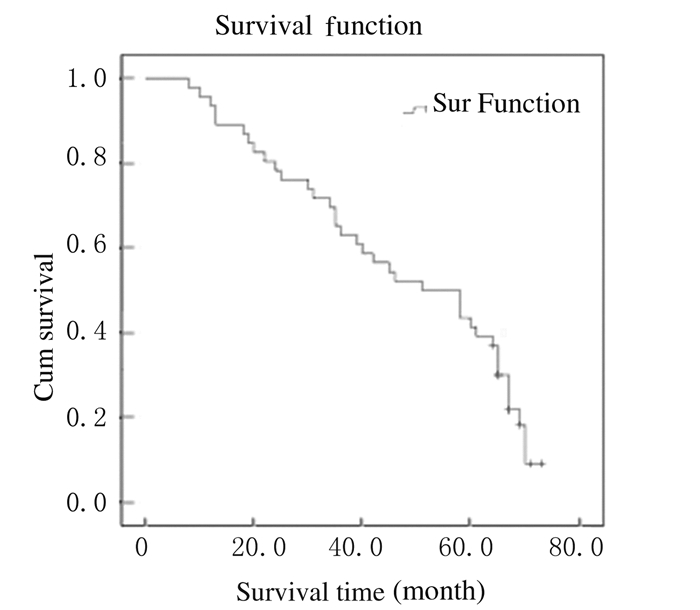
|
| 图 1 46例结肠直肠癌患者总体生存曲线 Figure 1 Overall survival curves of 46 patients with colorectal cancer |
|
|
| Factor | n | 5-yearsurvival rate (η/%) | Mediansurvival time(month) | 95%CI | P | |
| Lower limit | Upper limit | |||||
| Gender | ||||||
| Male | 28 | 35.7 | 58.00 | 29.48 | 86.52 | 0.34 |
| Female ‘ | 18 | 33.3 | 45.00 | 11.74 | 78.26 | |
| Age(year) | ||||||
| ≥60 | 23 | 34.8 | 58.00 | 43.91 | 72.09 | 0.92 |
| < 60 | 23 | 34.8 | 45.00 | 27.78 | 62.22 | |
| KRASgene | ||||||
| Wild type | 26 | 42.3 | 58.0 | 38.01 | 77.99 | 0.47 |
| Mutant type | 20 | 25.0 | 42.0 | 28.85 | 55.15 | |
| Tumor site | ||||||
| Right colon | 14 | 28.6 | 34.00 | 17.50 | 50.50 | 0.011 |
| Left colon | 32 | 37.5 | 58.00 | 45.55 | 70.45 | |
| Differentiation degree | ||||||
| Low | 13 | 30.8 | 42.00 | 12.06 | 71.95 | 0.21 |
| Medium/high | 33 | 36.7 | 58.00 | 39.99 | 76.01 | |
| Liver metastasis | ||||||
| Liver metastasis | 15 | 26.7 | 35.00 | 27.43 | 42.57 | 0.001 |
| Other | 31 | 38.7 | 58.00 | 47.09 | 68.91 | |
| Lung metastasis | ||||||
| Lung metastasis | 5 | 40.0 | 60.00 | 55.71 | 64.29 | 0.034 |
| Other | 41 | 34.1 | 45.00 | 25.14 | 54.87 | |
| Multiple metastases | ||||||
| Multiple metastases | 17 | 35.3 | 47.00 | 16.79 | 79.21 | 0.042 |
| Other | 29 | 34.5 | 59.00 | 20.32 | 89.68 | |

|
| 图 2 KRAS不同基因型结直肠癌患者总体生存曲线 Figure 2 Overall survival curves of colorectal cancer patients with different KRAS genotypes |
|
|
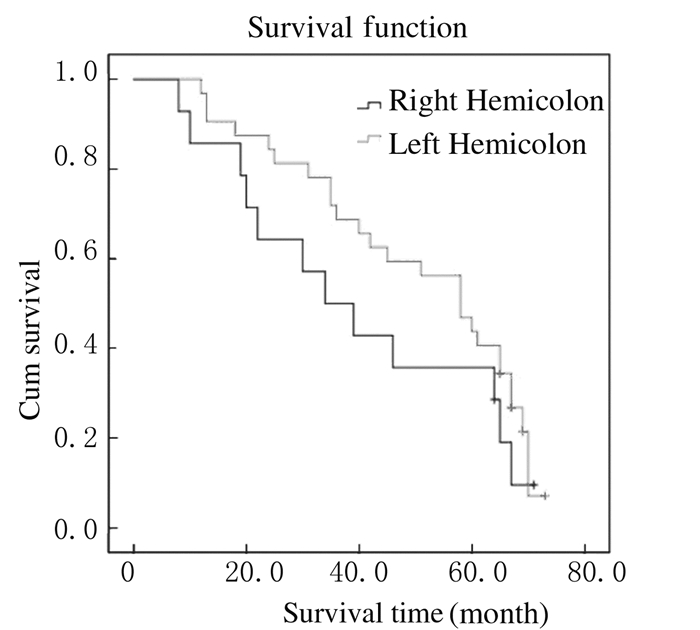
|
| 图 3 不同病变部位结直肠癌患者生存曲线 Figure 3 Survival curves ofpatients with colorectal cancer in different sites |
|
|
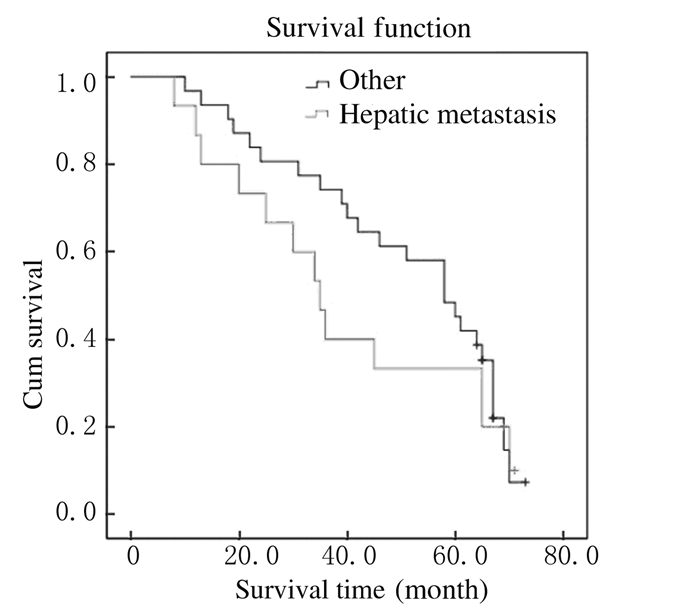
|
| 图 4 伴有或不伴有肝转移结直肠癌患者总体生存曲线 Figure 4 Overall survival curves of colorectal cancer patients with or without liver metastasis |
|
|
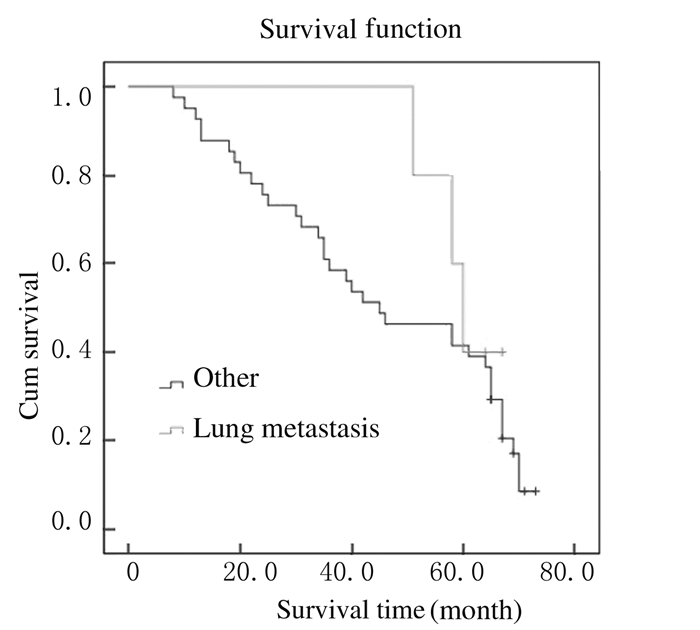
|
| 图 5 伴有或不伴有肺转移结直肠癌患者总体生存曲线 Figure 5 Overall survival curves of colorectal cancer patients with or without lung metastasis |
|
|
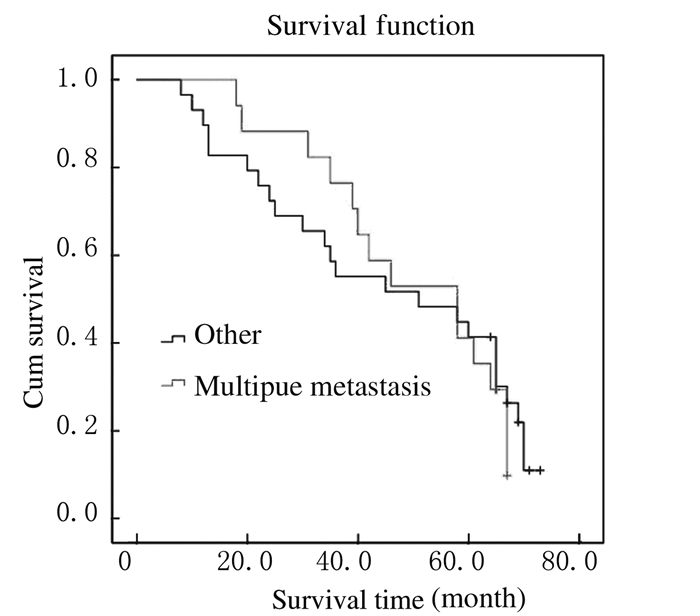
|
| 图 6 伴有或不伴有多发转移结直肠癌患者总体生存曲线 Figure 6 Overall survival curves of colorectal cancer patients with or without multiplemetastasis |
|
|
晚期转移性结直肠癌在确诊时只有40%的患者可直接或通过综合治疗后接受转移灶及原发灶切除的根治性手术[5-6],而其他的晚期结直肠癌患者则无机会接受根治性手术,对于这类患者有研究[7-8]指出:当原发灶存在完全或不完全肠梗阻、出血、穿孔等风险时,原发灶的切除可让患者改善生存、提高生活质量和减少并发症。而当患者只有轻微症状或无相关原发灶症状时,对于原发灶的范围选择、手术切除及时机和生存获益等仍存在争议,且原发灶切除对晚期结直肠癌患者长期预后和转移等的影响及监测方法仍未明确[9-10]。KRAS基因与细胞内信号转导相关。KRAS基因突变可以诱导肿瘤细胞的增殖、血管生成、侵袭和转移。有学者提出结直肠癌患者KRAS基因突变可以作为评价其预后及治疗的指标[11],当然也存在不同的看法[12]。原发灶切除后患者需进行KRAS基因突变检测,以指导后续靶向治疗。因此本文作者对Ⅳ期结直肠癌轻微症状原发灶切除患者KRAS基因突变率进行检测,分析其与患者预后和临床病理特征的关系,为Ⅳ期结直肠癌症状轻微患者原发灶切除后的临床治疗及监测提供依据和思路。
本研究结果显示:本组Ⅳ期患者中位生存时间较长,达到39.6个月,随访至2016年1月仍有部分患者存活;左半结肠癌和右半结肠癌患者生存期均有延长趋势,右半结肠癌患者比左半结肠癌患者的生存质量差,组间比较差异有统计学意义;提示对于轻微症状的Ⅳ期结直肠癌患者行原发灶切除后,其存在生存优势,生活质量有所改善。这与既往研究[13]支持手术切除原发灶能够带来生存上的获益、改善生活质量等结果相似。同时,本研究结果显示:癌细胞的转移对结直肠患者的术后预后具有明显的影响,肝转移、肺转移和多发转移与结直肠癌患者术后预后不良关系密切。同时患者生存期的延长也可能得益于最近10年来奥沙利铂、依立替康、西妥昔单抗、贝伐珠单抗和瑞戈非尼等靶向药物在晚期结直肠癌患者中的应用。研究[14-15]报道:结直肠癌患者原发灶切除并未提高其总生存率,反而增加并发症发生率,这可能与多数研究为回顾性、原发灶切除后患者的生存获益因难、选择的偏倚和根治性切除占一定比例等有关。
KRAS基因突变与肿瘤原发部位和多发转移有关,本研究结果提示右半结肠癌患者和多发转移的患者更易发生KRAS基因突变。另外,KRAS基因突变与患者其他临床病理参数如肿瘤分化程度、年龄和性别等均无关联。本研究与既往研究报道[16]结果一致。本研究结果显示:KRAS基因野生型和突变型原发灶切除的Ⅳ期结直肠癌患者总体生存期比较差异无统计学意义,但KRAS野生型患者显示了可能的潜在优势,这符合KRAS基因突变并不具有结肠直肠癌患者手术预后的预测价值的观点[17]。研究[18]提示:KRAS基因突变对结肠直肠癌患者手术预后存在一定的影响,这可能与检测样本量、方法和环境等影响KRAS基因突变检测率,导致结果出现偏差有关[19]。本研究结果显示:肿瘤原发部位和转移部位与患者预后不良关系密切。
根据目前最新研究进展,结直肠癌患者检测KRAS的同时检测NRAS或BRAF基因,更有利于制订出治疗方案,使更多的结直肠癌患者获得更好的收益[20]。由于实验条件限制,本组患者只能进行KRAS检测。
综上所述,根据目前得出的结果,Ⅳ期结直肠癌轻微症状患者原发灶切除后生存期延长,右半结肠癌预后比左半结肠癌差,转移部位影响预后,结直肠癌患者KRAS基因变异与肿瘤原发部位和肿瘤的多发转移有密切关联,虽然KRAS基因突变与患者手术预后关联性不能确定,但患者原发灶切除带来生存优势有获益的潜在趋势,需要扩大样本量,同时检测KRA、NRAS和BRAF基因状况,进行进一步研究。
| [1] | ShidaD, HamaguchiT, OchiaiH. Prognostic impact of palliative primary tumor resection for unresectable stage 4 colorectal cancer:Using a propensity score analysis[J]. Ann Surg Oncol, 2016, 23(11): 1–7. |
| [2] | Winner M, Mooney SJ, Hershman DL, et al. Incidence and pre-dictors of bowel obstruction in elderly patients with stage Ⅳ colon cancer:a population-based cohort study[J]. JAMA Surg, 2013, 148(8): 715–722. DOI:10.1001/jamasurg.2013.1 |
| [3] | Ishihara S, NishikawaT, TanakaT, et al. Benefit of primary tumor resection in stage Ⅳ colorectal cancer with unresectable metastasis:a multicenter retrospective study using a propensity score analysis[J]. Int J Colorectal Dis, 2015, 30(6): 807–812. DOI:10.1007/s00384-015-2228-4 |
| [4] | Kim MJ, Lee HS, Kim JH, et al. Different metastatic pattern according to the KRAS mutational status and site-specificdiscordance of KRAS status in patients with colorectal cancer[J]. BMC Cancer, 2012, 12: 347. DOI:10.1186/1471-2407-12-347 |
| [5] | Kim YW, Kim IY. The role of surgery for asymptomatic primarytumors in unresectable stage Ⅳ colorectal cancer[J]. Ann Coloproctol, 2013, 29(2): 44–54. DOI:10.3393/ac.2013.29.2.44 |
| [6] | Yang TX, Billah B, Morris DL, et al. Primary colorectal cancer palliative resection:early results after laparoscopic colectomy and open system review and meta-analysis[J]. Colorectal Dis, 2013, 15(8): 407–419. DOI:10.1111/codi.2013.15.issue-8 |
| [7] | TurnerN, TranB, TranPV, et al. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival[J]. Clin Colorectal Cancer, 2015, 10(3): 185–191. |
| [8] | Ikoma N, Rodriguez-Bigas MA. Managing the primary tumor with unresectable synchronous colorectal metastases[J]. Curr Colorectal Cancer Rep, 2016, 12(3): 170–179. DOI:10.1007/s11888-016-0322-9 |
| [9] | Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage Ⅳ colorectal cancer receiving combination chemotherapy without surgery as initial treatment[J]. J Clin Oncol, 2009, 27(20): 3379–3384. DOI:10.1200/JCO.2008.20.9817 |
| [10] | Ahmed S, Shahid RK, Leis A, et al. Should noncurative resection of the primary tumour be performed in patients with stage iv colorectal cancer? A systematic review and meta-analysis[J]. Curr Oncol, 2013, 20(5): e420–e441. DOI:10.3747/co.20.1469 |
| [11] | Hu J, Yan WY, Xie L, et al. Coexistence of MSI with KRAS mutation is associated with worse prognosis in colorectal cancer[J]. Medicine(Baltimore), 2016, 95(50): e5649. |
| [12] | Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage Ⅱ and Ⅲ resected colon cancer:results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial[J]. J Clin Oncol, 2010, 28(3): 466–474. DOI:10.1200/JCO.2009.23.3452 |
| [13] | Verhoef C, de Wilt JH, Burger JW, et al. Surgery of the primaryin stage Ⅳ colorectal cancer with unresectable metastases[J]. Eur J Cancer, 2011, 47(Suppl 3): S61–S66. |
| [14] | Evans MD, Escofet X, Karandikar SS, et al. In colorectal cancer management of patients with resection and non surgical strategy results in the late[J]. World J Surg Oncol, 2009, 7(1): 28. DOI:10.1186/1477-7819-7-28 |
| [15] | 苏燕燕, 杨羽中, 董兵, 等. 右半结肠癌小淋巴结的检出及对病理分期的影响[J]. 解放军医学杂志, 2016, 41(10): 879–890. DOI:10.11855/j.issn.0577-7402.2016.10.17 |
| [16] | Modest DP, Stintzing S, Laubender RP, et al. Clinical characterization of patients with metastatic colorectal cancer depending on the KRAS status[J]. Anticancer Drugs, 2011, 22(9): 913–918. DOI:10.1097/CAD.0b013e3283493160 |
| [17] | Gonzalez-Aguilera JJ, Oliart S, Azcoita MM, et al. Simultaneous mutations in K-ras and TP53 are indicative of poor prognosis in sporadic colorectal cancer[J]. Am J Clin Oncol, 2004, 27(1): 39–45. DOI:10.1097/01.coc.0000045920.49210.7A |
| [18] | Krzysztof R, Bogdan Z, Wojciech JO, et al. Impact of specific KRAS mutation in exon 2 on clinical outcome of chemotherapy-and radiotherapy-treated colorectal adenocarcinoma patients[J]. Mol Diagn Ther, 2014, 18(5): 559–566. DOI:10.1007/s40291-014-0107-2 |
| [19] | Bazan V, Migliavacca M, Zanna I, et al. Specific codon 13 K-ras mutations are predictive of clinical outcome in colorectal cancer patients, whereas codon 12 K-ras mutations are associated with mucinous histotype[J]. Ann Oncol, 2002, 13(9): 1438–1446. DOI:10.1093/annonc/mdf226 |
| [20] | Zhang J, Zheng J, Yang YH, et al. Molecular spectrum of KRAS, NRAS, BRAF and PIK3 mutations in Chinese colorectal cancer patients:analysis of 1110 cases[J]. Sci Rep, 2015, 5: 18678. |
 2017, Vol. 43
2017, Vol. 43


