扩展功能
文章信息
- 石俊忠, 庄建彬, 张传山, 刘贤明, 孔大陆
- SHI Junzhong, ZHUANG Jianbin, ZHANG Chuanshan, LIU Xianming, KONG Dalu
- β-tubulin Ⅲ在结肠腺癌组织中的表达及其临床意义
- Expression of β-tubulin Ⅲ in colon adenocarcinoma tissue and its clinical significance
- 吉林大学学报(医学版), 2017, 43(04): 770-775
- Journal of Jilin University (Medicine Edition), 2017, 43(04): 770-775
- 10.13481/j.1671-587x.20170421
-
文章历史
- 收稿日期: 2017-02-04
2. 天津市第三中心医院病理科, 天津 300170;
3. 天津医科大学肿瘤医院肿瘤内科, 天津 300060;
4. 天津医科大学肿瘤医院结直肠肿瘤科, 天津 300060
2. Department of Pathology, Tianjin Third Central Hospital, Tianjin 300170, China;
3. Department of Oncology Cancer Hospital, Tianjin Medical University, Tianjin 300060, China;
4. Department of Colorectum Oncology, Cancer Hospital, Tianjin Medical University, Tianjin 300060, China
结直肠癌是世界高死亡率肿瘤之一,全球发病率和死亡率呈逐年上升趋势[1]。微管作为抗肿瘤的靶点已成为研究的热点,而抗微管类药物是目前临床上常用的抗肿瘤药物。β-tubulin Ⅲ作为β微管蛋白中最重要的亚型之一,参与细胞的物质运输、有丝分裂和形状改变等活动,在细胞的定向迁移、增殖分化及肿瘤细胞的发生过程中有十分重要的作用。研究[2]表明:微管蛋白亚型中,β-tubulin Ⅲ是微管作用药物耐药的唯一标志物。目前国内外关于β-tubulin Ⅲ在结肠腺癌组织中的表达及其意义的报道很少。本研究通过免疫组织化学方法检测β-tubulin Ⅲ在结肠癌组织中的表达,探讨β-tubulin Ⅲ的表达与结肠癌患者临床病理特征的关系。
1 资料与方法 1.1 临床资料收集2013年6月—2016月6月在天津医科大学肿瘤医院和天津市第三中心医院行结肠腺癌根治术的111例患者的结肠腺癌组织。其中男性55例,女性56例,平均年龄(65.48±6.72) 岁;发生淋巴结转移56例;高分化18例,中分化68例,低分化25例;临床分期Ⅰ期18例,Ⅱ期35例,Ⅲ期46例,Ⅳ期12例;死亡9例,复发5例。
1.2 诊断标准纳入标准:① 所有纳入患者术后均经组织病理学检查确诊为结肠腺癌。② 切除的组织样本依据国际抗癌联盟制定的第7版TNM分期系统[3]进行病理学诊断。病理学诊断采用双盲法阅片判断,由2名具有高级职称的医师在光学显微镜下观察,进行分析,取结果一致者;若结果不一致,再请主任医师参与会诊。③ 术后随访数据完整。④ 组织标本获取经家属签署知情同意书。排除标准:① 术前接受过放化疗或生物治疗的患者;② 术后病理诊断不明者;③ 并发其他恶性肿瘤者。
1.3 主要试剂和仪器鼠抗人β-tubulin Ⅲ单克隆抗体一抗购自美国Santa Cruz公司,鼠抗人单克隆抗体CK一抗及通用型羊抗鼠抗体二抗均购自北京中杉金桥生物技术有限公司,免疫组织化学试剂盒购自福州迈新生物技术有限公司。RM2235型石蜡切片机购于德国莱卡公司,BMJ-Ⅲ型包埋机购于江苏省常州中威电子仪器厂,BX51显微镜购于日本Olympus公司。
1.4 免疫组织化学染色所有结肠腺癌组织均经4%中性甲醛固定24h,进行石蜡包埋,包埋的蜡块用切片机切成厚4 μm的切片。切片置于65℃恒温箱内4h,二甲苯中脱蜡,在酒精中脱二甲苯,行免疫组织化学染色。免疫组织化学染色按照试剂盒说明书进行,PBS代替一抗作为阴性对照。3% H2O2甲醇液中10 min,PBS冲洗5 min×5次,加封闭液孵育10 min。倒掉液体加入一抗(1:50),4℃过夜。PBS洗5 min×5次,加抗生物素化标记的二抗孵育10 min,PBS洗5 min×5次。PBS洗5 min×5次,加酶标记的二抗,室温孵育45 min,PBS洗5 min×5次。DAB显色、水洗、复染、脱水、透明、封片。
1.5 免疫组织化学染色结果判定标准每张切片选取5个视野,采用双盲法阅片法分析阳性细胞数占细胞总数的比例。<5%为0分,5%~25%为1分,26%~50%为2分,51%~75%为3分,>75%为4分。染色强度:无着色计阴性0分,浅黄色计弱阳性1分,棕黄色计中等阳性2分,棕褐色计强阳性3分。2项指标乘积0分为阴性,1~5分为弱阳性,6~8分为中阳性,9~12分为强阳性。
1.6 统计学分析采用SPSS 19.0统计软件进行统计学分析。浸润前沿组和非前沿组患者年龄以x±s表示,组间比较采用t检验;β-tubulin Ⅲ阳性表达率和患者的定性资料(性别、淋巴结转移、肿瘤分化程度、临床分期、复发和死亡)采用频数和百分率表示,组间比较采用χ2检验或Fisher检验。以P < 0.05为差异有统计学意义。
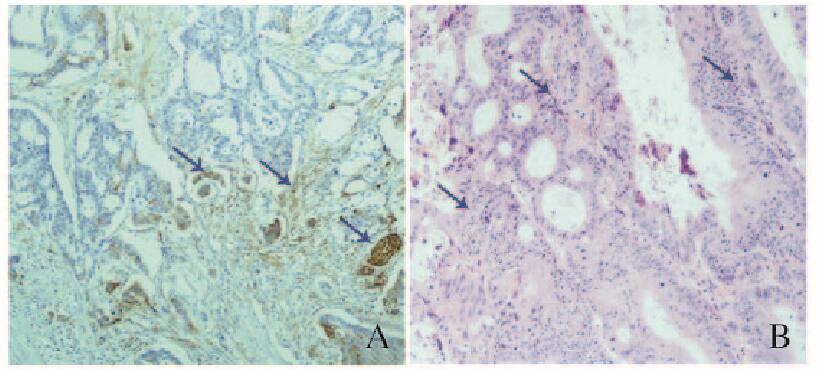
2 结果 2.1 结肠腺癌组织中β-tubulin Ⅲ阳性表达情况根据β-tubulin Ⅲ阳性瘤细胞的分布情况,将结肠腺癌组织分为浸润前沿组(72例)和非浸润前沿组(39例)。浸润前沿组中,β-tubulin Ⅲ在肿瘤浸润方向前沿的肿瘤细胞中呈阳性表达,其他区域呈阴性或者散在阳性表达,阳性细胞远离肿瘤主体,偏心性分布于肿瘤浸润前沿位置,肿瘤和间质的交界处部分阳性细胞呈梭形(图 1A,见封三);非浸润前沿组β-tubulin Ⅲ阳性癌细胞弥漫分布于浸润性癌巢中(图 1B,见封三)。
|
| A: Front group; B: Non-front group. The arrows referred to the positive cells. 图 1 2组患者结肠腺癌组织中β-tubulin Ⅲ阳性表达(×400) Figure 1 Positive expressions of β-tubulin Ⅲin cancer tissue of patients with colon adenocarcinoma in two groups (×400) |
|
|
2组患者在性别构成、年龄、淋巴结转移、临床分期、死亡和复发等方面比较差异无统计学意义(P > 0.05)。2组患者癌组织中β-tubulin Ⅲ阳性表达率比较差异有统计学意义(χ2=8.76,P=0.01)。肿瘤低中分化病例多见于浸润前沿组,而高分化病例多见于非浸润前沿组,组间比较差异有统计学意义(χ2=6.88,P=0.032)。见表 1。
| Group | n | Sex [n(η/%)] | Age(years) | β-tubulinⅢ expression[n(η/%)] | n | Lymph node metastasis[n(η/%)] | Tumor differentiation [n(η/%)] | Death [n(η/%)] | n | Clinical stage[n(η/%)] | Recurrence [n(η/%)] | |||||||||||||||
| Male | Female | Negative | Weakly positive | Positive | Strongly positive | Yes | No | High | Moderate | Low | Yes | No | Ⅰ | Ⅱ | Ⅲ | Ⅳ | Yes | No | ||||||||
| Non-front | 39 | 20(51.28) | 19(48.72) | 62.36±11.18 | 6(15.38) | 2(5.13) | 23(58.97) | 8(20.51) | 39 | 19(48.72) | 20(51.28) | 11(28.21) | 19(48.72) | 9(23.08) | 4(10.26) | 35(89.74) | 39 | 8(20.51) | 11(28.21) | 18(46.15) | 2(5.13) | 2(2.56) | 37(97.44) | |||
| Front | 72 | 35(48.61) | 37(51.39) | 64.24±10.26 | 0(0.00) | 8(11.11) | 42(58.33) | 22(30.56) | 72 | 37(51.39) | 35(48.61) | 7(9.72) | 49(68.06) | 16(22.22) | 5(6.94) | 67(93.06) | 72 | 10(13.89) | 24(33.33) | 28(38.89) | 10(13.89) | 3(1.39) | 69(98.61) | |||
| t/χ2 | 1.12 | -0.88 | 8.76 | 0.00 | 0.98 | 0.23 | 3.01 | 0.21 | ||||||||||||||||||
| P | 0.28 | 0.29 | 0.01 | 0.98 | 0.03 | 0.72 | 0.39 | 0.99 | ||||||||||||||||||
不同分化程度结肠腺癌患者癌组织中β-tubulin Ⅲ阳性表达率比较差异有统计学意义(χ2=5.74,P=0.04),而结肠腺癌患者癌组织中β-tubulin Ⅲ的阳性表达率与患者性别、淋巴结转移、临床分期、死亡和复发无关联(P > 0.05)。
39例非浸润前沿组患者中,无淋巴结转移患者19例(β-tubulin Ⅲ阴性表达5例、弱阳性表达1例、中阳性表达12例和强阳性表达1例),有淋巴结转移患者20例(β-tubulin Ⅲ阴性表达1例、弱阳性表达1例、中阳性表达11例、强阳性表达7例)。非浸润前沿组患者癌组织中β-tubulin Ⅲ的阳性表达率与淋巴结转移有关(χ2=6.02,P=0.05)。见表 2。
| Clinicopathological feature | n | Expression of β-tubulin Ⅲ | χ2 | P | |||
| Negative | Weakly positive | Positive | Strongly positive | ||||
| Gender | |||||||
| Male | 55 | 4(50.00) | 5(45.45) | 30(48.39) | 16(53.33) | 0.03 | 0.78 |
| Female | 56 | 4(50.00) | 6(54.55) | 32(51.61) | 14(46.67) | ||
| Lymph node metastasis | |||||||
| Yes | 56 | 2(28.57) | 5(45.45) | 32(53.33) | 17(50.00) | 0.00 | 0.75 |
| No | 55 | 5(71.43) | 6(54.55) | 28(46.67) | 17(50.00) | ||
| Tumor differentiation | |||||||
| High | 18 | 3(50.00) | 1(10.00) | 12(18.46) | 2(6.67) | 5.74 | 0.04 |
| Moderate | 68 | 1(16.67) | 6(60.00) | 42(64.62) | 19(63.33) | ||
| Low | 25 | 2(33.33) | 3(30.00) | 11(16.92) | 9(30.00) | ||
| Death | |||||||
| Yes | 9 | 0(0.00) | 1(10.00) | 5(7.69) | 3(10.00) | 0.07 | 0.94 |
| No | 102 | 6(100.00) | 9(90.00) | 60(92.31) | 27(90.00) | ||
| Clinical stage | |||||||
| Ⅰ | 18 | 1(16.67) | 3(30.00) | 10(15.38) | 4(13.33) | 0.00 | 0.60 |
| Ⅱ | 35 | 4(66.67) | 1(10.00) | 22(33.85) | 8(26.67) | ||
| Ⅲ | 46 | 1(16.67) | 5(50.00) | 26(40.00) | 14(46.67) | ||
| Ⅳ | 12 | 0(0.00) | 1(10.00) | 7(10.77) | 4(13.33) | ||
| Recurrence | |||||||
| Yes | 5 | 0(0.00) | 1(8.33) | 2(3.28) | 2(6.06) | 0.07 | 0.53 |
| No | 106 | 5(100.0) | 11(91.67) | 59(96.72) | 31(93.94) | ||
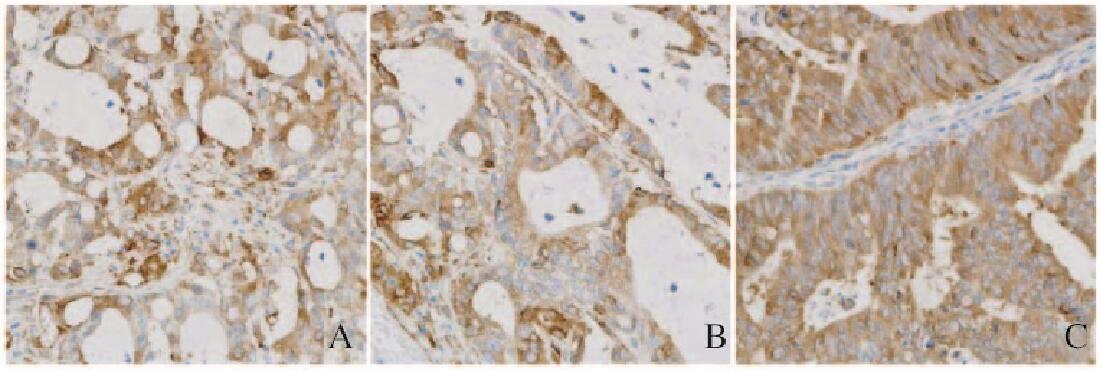
β-tubulin Ⅲ主要表达于结肠腺癌细胞胞质中,呈黄色或棕黄色颗粒。β-tubulin Ⅲ阳性表达与结肠腺癌分化程度有关联,高分化结肠腺癌组织中β-tubulin Ⅲ阳性表达细胞明显多于低分化及中分化结肠腺癌组织,以低分化结肠腺癌组织中最少。见图 2(封三)。
|
| A: Low differentiation; B: Moderate differentiation; C: High differentiation. 图 2 不同分化程度结肠腺癌组织中β-tubulin Ⅲ阳性表达(×400) Figure 2 Positive expressions of β-tubulin Ⅲ in colon adenocarcinoma tissues with different differentiation degrees (×400) |
|
|
结肠癌作为常见的恶性肿瘤之一,在中国的发病率和病死率逐年升高[1]。微管是细胞骨架的基本组成部分,在细胞增殖、信号传递及肿瘤发展过程中发挥着重要作用。研究[4-5]表明:以微管为靶点是抗肿瘤行之有效的途径之一,抗微管类靶向药物可促使肿瘤细胞凋亡。β-tubulin Ⅲ又被称为TUBB3,是β微管蛋白家族的重要亚型之一,在肿瘤发展过程中起重要作用。本研究结果表明:β-tubulin Ⅲ的阳性表达率在不同性别、年龄、淋巴结转移、临床分期、死亡和复发结肠腺癌患者间比较差异无统计学意义,但与肿瘤分化程度有关联,在非前沿组患者中,β-tubulin Ⅲ的阳性表达与淋巴结转移有关。
研究[6]表明:细胞骨架结构的稳定性和动力性与恶性肿瘤的浸润程度有密切关联。研究[7]表明:β-tubulin Ⅲ的表达与肿瘤浸润深度有关。β-tubulin Ⅲ在胃癌组织中的表达率稍高于癌旁组织,但组间比较差异无统计学意义。β-tubulin Ⅲ的过表达可作为诊断前列腺癌进展的独立预测因子[8]。β-tubulin Ⅲ的表达可作为局部晚期头颈部鳞癌患者化疗疗效预测指标和不良预后的生物标志,阳性表达患者化疗疗效差[9]。β-tubulin Ⅲ高表达与乳腺癌的侵袭性肿瘤特征有关,尤其是乳腺癌的不良特征[10]。β-tubulin Ⅲ过表达与卵巢上皮癌患者的无进展生存期[11]和结直肠癌患者的左侧肿瘤定位[12]有关联。不同于以往的研究,本研究首先根据β-tubulin Ⅲ阳性瘤细胞在结肠癌组织的分布情况,将所有结肠腺癌组织分为浸润前沿组和非浸润前沿组,浸润前沿组β-tubulin Ⅲ阳性细胞远离肿瘤主体,部分阳性细胞呈梭形,而非浸润前沿组β-tubulin Ⅲ阳性细胞弥漫分布于浸润性癌巢中;结肠腺癌组织中β-tubulin Ⅲ表达与肿瘤的分化有关,高分化结肠腺癌组织多见于非前沿组。β-tubulin Ⅲ微管蛋白可用作鉴定非黏液性肺腺癌早期浸润灶的标记物[13]。本研究结果表明:β-tubulin Ⅲ可作为评估结肠腺癌的分化水平的重要指标。
为了进一步验证结论,本研究又分析了结肠腺癌组织中不同β-tubulin Ⅲ的表达与患者临床特征的关系,结果同样表明:不同分化程度结肠癌组织中β-tubulin Ⅲ的阳性表达率比较差异有统计学意义。研究[7-8]表明:胃癌或前列腺癌组织中β-tubulin Ⅲ的表达与患者的性别、年龄、肿瘤大小、部位、Borrmann分型和TNM分期均无明显相关性,与本研究的结果相一致。不同于胃癌或者前列腺癌组织,结肠腺癌组织中β-tubulin Ⅲ的阳性表达与肿瘤分化程度有关。研究[14]表明:卵巢癌患者中β-tubulin Ⅲ过表达与患者分期、有无腹水、淋巴结转移等临床特征有密切关联。无淋巴结转移的非小细胞肺癌患者β-tubulin Ⅲ的表达水平明显高于有淋巴结转移的患者,Ⅰ/Ⅱ期和Ⅳ期的患者中β-tubulin Ⅲ的表达明显高于Ⅲ期患者[15]。食管鳞状细胞癌患者β-tubulin Ⅲ的阳性表达与性别有关联[16]。本研究结果表明:非浸润前沿组有淋巴结转移的患者β-tubulin Ⅲ阳性表达强度较无淋巴结转移患者更强,而在浸润前沿组患者中β-tubulin Ⅲ的表达强度却与淋巴结转移无关联。对于非前沿浸润的结肠癌患者,检测到高分化的肿瘤要考虑淋巴结转移的潜在可能性。
另外,免疫染色结果表明:β-tubulin Ⅲ在结肠腺癌组织中阳性表达定位于细胞质中,与其在胃癌组织中表达定位相一致[17]。目前,大量研究[18-19]报道:β-tubulin Ⅲ与抗肿瘤药物耐药性密切相关,可通过影响患者对药物的反应而影响其预后。β-tubulin Ⅲ还可以通过信号通路参与肿瘤的发展和细胞的凋亡[20-21]。β-tubulin Ⅲ可通过参与Erk和Akt信号通路,进而调节结肠癌CT26细胞凋亡[20]。PTEN/AKT信号可通过ATP和Caspase-3信号通路影响TUBB3和TOP2A基因的表达,减少细胞生长并诱导人乳腺癌MCF-7细胞的凋亡[21]。总之,β-tubulin Ⅲ在肿瘤中的表达与调控机制,还需大量的实验进行验证。
综上所述,结肠腺癌组织中β-tubulin Ⅲ的阳性表达率与肿瘤的分化程度有关,非前沿组患者β-tubulin Ⅲ的表达与淋巴结转移有密切关联。高分化非前沿结肠腺癌患者要考虑淋巴结转移的可能性。β-tubulin Ⅲ的阳性表达是评估结肠腺癌的重要指标,对制订个体化治疗方案及预后分析具有重要意义。
| [1] | Zhu J, Tan Z, Hollis-Hansen K, et al. Epidemiological trends in colorectal cancer in China:An ecological study[J]. Dig Dis Sci, 2017, 62(1): 235–243. DOI:10.1007/s10620-016-4362-4 |
| [2] | Verdier-Pinard P, Wang F, Martello L, et al. Analysis of tubulin isotypes and mutations from taxol-resistant cells by combined isoelectrofocusing and mass spectrometry[J]. Biochemistry, 2003, 42(18): 5349–5357. DOI:10.1021/bi027293o |
| [3] | 权继传, 解亦斌, 田艳涛. 国际抗癌联盟胃癌TNM分期系统第七版解读[J]. 中华诊断学电子杂志, 2014, 2(1): 59–61. |
| [4] | Fife CM, McCarroll JA, Kavallaris M. Movers and shakers:cell cytoskeleton in cancer metastasis[J]. Br J Pharmacol, 2014, 171(24): 5507–5523. DOI:10.1111/bph.2014.171.issue-24 |
| [5] | Klute K, Nackos E, Tasaki S, et al. Microtubule inhibitor-based antibody-drug conjugates for cancer therapy[J]. Onco Targets Ther, 2014, 7: 2227–2236. |
| [6] | Portyanko A, Kovalev P, Gorgun J, et al. β Ⅲ-tubulin at the invasive margin of colorectal cancer:possible link to invasion[J]. Virchows Arch, 2009, 454(5): 541. DOI:10.1007/s00428-009-0764-4 |
| [7] | 曹友红, 马平, 王福根, 等. β-tubulin Ⅲ在胃癌中的表达及临床意义[J]. 新医学, 2014(7): 444–447. |
| [8] | Tsourlakis MC, Weigand P, Grupp K, et al. β Ⅲ-tubulin overexpression is an independent predictor of prostate cancer progression tightly linked to ERG fusion status and PTEN deletion[J]. Am J Pathol, 2014, 184(3): 609–617. DOI:10.1016/j.ajpath.2013.11.007 |
| [9] | Koh Y, Kim TM, Jeon YK, et al. Class Ⅲ-tubulin, but not ERCC1, is a strong predictive and prognostic marker in locally advanced head and neck squamous cell carcinoma[J]. Ann Oncol, 2009, 20(8): 1414–1419. DOI:10.1093/annonc/mdp002 |
| [10] | Lebok P, Öztürk M, Heilenk tter U, et al. High levels of class Ⅲ β-tubulin expression are associated with aggressive tumor features in breast cancer[J]. Oncol Lett, 2016, 11(3): 1987–1994. |
| [11] | Du J, Li B, Fang Y, et al. Overexpression of class Ⅲ β-tubulin, Sox2, and nuclear Survivin is predictive of taxane resistance in patients with stage Ⅲ ovarian epithelial cancer[J]. BMC Cancer, 2015, 15(1): 536–547. DOI:10.1186/s12885-015-1553-x |
| [12] | Melling N, Tsaklakidis AM, Simon R, et al. β Ⅲ-tubulin overexpression is linked to left-sided tumor localization and nuclear β-catenin expression in colorectal cancer[J]. Cancer Treat Commun, 2015, 6(4): 96–102. |
| [13] | 杨清海, 陈惠玲, 曾德华, 等. 非黏液型肺腺癌中β-Tubulin-Ⅲ作为早期浸润灶标志物的应用[J]. 临床与实验病理学杂志, 2016, 32(7): 753–756. |
| [14] | Izutsu N, Maesawa C, Shibazaki M, et al. Epigenetic modification is involved in aberrant expression of class Ⅲ beta-tubulin, TUBB3, in ovarian cancer cells[J]. Int J Oncol, 2008, 32(6): 1227–1235. |
| [15] | Zou ZQ, Du YY, Sui G, et al. Expression of TS, RRM1, ERCC1, TUBB3 and STMN1 Genes in Tissues of Non-small Cell Lung Cancer and its Significance in Guiding Postoperative Adjuvant Chemotherapy[J]. Asian Pac J Cancer Prev, 2015, 16(8): 3189–3194. DOI:10.7314/APJCP.2015.16.8.3189 |
| [16] | 杨立伟, 韩青松, 栾艳超, 等. 食管鳞癌患者ERCC1、TYMS、TUBB3的表达特征及其与化疗效果的相关性分析[J]. 基因组学与应用生物学, 2016(11): 2941–2945. |
| [17] | Lu M, Gao J, Wang X, et al. Expressions of thymidylate synthase, thymidine phosphorylase, class Ⅲ β-tubulin, and excision repair cross-complementing group 1 predict response in advanced gastric cancer patients receiving capecitabine plus paclitaxel or cisplatin[J]. Chin J Cancer Res, 2011, 23(4): 288–294. DOI:10.1007/s11670-011-0288-8 |
| [18] | Aldonza MBD, Hong JY, Alinsug MV, et al. Multiplicity of acquired cross-resistance in paclitaxel-resistant cancer cells is associated with feedback control of TUBB3 via FOXO3a-mediated ABCB1 regulation[J]. Oncotarget, 2016, 7(23): 34395–34419. DOI:10.18632/oncotarget.v7i23 |
| [19] | 钟科. TUBB3和TOP2A与晚期乳腺癌患者临床病理特征及与紫杉类联合蒽环类药物治疗疗效的关系[J]. 第三军医大学学报, 2016, 38(14): 1659–1663. |
| [20] | Ming X, Yi T, Chen WW, et al. Tubb3 regulation by the Erk and Akt signaling pathways:a mechanism involved in the effect of arginine ADP-ribosyltransferase 1(Art1) on apoptosis of colon carcinoma CT26 cells[J]. Tumor Biol, 2016, 37(2): 2353–2363. DOI:10.1007/s13277-015-4058-y |
| [21] | Yang Z, Liu Y, Shi C, et al. Suppression of PTEN/AKT signaling decreases the expression of TUBB3 and TOP2A with subsequent inhibition of cell growth and induction of apoptosis in human breast cancer MCF-7 cells via ATP and caspase-3 signaling pathways[J]. Oncol Rep, 2017, 37(2): 1011–1019. |
 2017, Vol. 43
2017, Vol. 43


