扩展功能
文章信息
- 赵利军, 李开济, 吴静, 门秀丽
- ZHAO Lijun, LI Kaiji, WU Jing, MEN Xiuli
- 缺血后处理对大鼠肢体缺血再灌注后肾细胞凋亡的抑制作用
- Inhibitory effect of ischemic postconditioning on apoptosis of renal cells after limb ischemia reperfusion in rats
- 吉林大学学报(医学版), 2017, 43(04): 725-728
- Journal of Jilin University (Medicine Edition), 2017, 43(04): 725-728
- 10.13481/j.1671-587x.20170412
-
文章历史
- 收稿日期: 2016-09-22
肢体缺血再灌注(limb ischemia reperfusion,LIR)损伤是临床外科常见的现象,随着缺血肢体血运的重建,多种有害因子播散性地循环全身,常影响机体的功能[1]。肾脏是维持机体内环境稳定的重要器官,实验[2-3]证明:严重的LIR可致急性肾功能衰竭,其中肾组织细胞的大量凋亡是重要机制之一。缺血后处理(ischemic postconditioning,I-postC)是在正式再灌注前进行反复短暂的缺血-再灌注的一种预处理方法[4],对大鼠LIR后肾损伤的影响尚不完全明确。本研究从细胞凋亡的角度观察LIR后肾脏功能和结构的变化,探讨I-postC对远位器官的保护作用。
1 材料与方法 1.1 实验动物和分组30只健康清洁级雄性Wistar大鼠,体质量200~250g,购自河北联合大学实验动物中心,动物合格证号:SCXK京2009-0004。大鼠随机分为3组(每组10只):对照组、缺血再灌注组(IR组)和缺血后处理加再灌注组(I-postC组)。术前24h禁食,饮水自由。
1.2 模型制备和指标检测以本室常规应用的止血带法复制大鼠LIR模型[1],使用标准化弹性的橡皮带环绕结扎大鼠双后肢根部阻断血流4h,然后松解橡皮带恢复血流灌注4h。对照组大鼠松弛结扎双后肢不阻断血流,I-postC组大鼠在血流再灌注前,先行缺血5 min-再灌注5 min,重复3次,再恢复血流灌注4h。各组大鼠于再灌注4h时经腹主动脉取血, 血液经3500 r·min-1离心15 min,所得血浆标本分装在干净的Eppendorf管中,-70℃保存备用。采用全自动生化分析仪分光光度法测定各组大鼠血浆肌酐(Cr)、尿素氮(BUN)和C反应蛋白(CRP)水平。剖腹取出一侧肾脏,横断面切取组织块(长约4mm),迅速投入10%甲醛溶液中固定,次日更换固定液,石蜡包埋后切片,TUNEL染色光镜下检测细胞凋亡情况;免疫组织化学法检测凋亡相关蛋白Bcl-2及Bax的表达情况;采用BI2000医学图像分析系统对各组大鼠肾组织切片进行分析,光镜下以相同外部条件摄取图像(10×20),每张切片随机选择6个视野,计算平均吸光度(A)值,作为蛋白定量表达结果。手术中快速切取约米粒大小肾皮质组织块,立即投入冷4%戊二醛-磷酸缓冲液,切成1mm×1mm×1mm的小块预固定,后转入2.5%戊二醛中固定,经丙酮脱水、环氧树脂618包埋、超薄切片、染色后在透射电镜下观察肾组织的超微结构。
1.3 统计学分析采用SPSS13.0统计软件进行统计学分析。各组大鼠血浆Cr、BUN和CRP水平,肾组织中Bcl-2和Bax蛋白表达水平以x± s表示,组间样本均数比较采用单因素方差分析(One-Way ANOVA)。以P<0.05为差异有统计学意义。
2 结果 2.1 各组大鼠血浆中Cr、BUN和CRP水平与对照组比较,IR组和I-postC组大鼠血浆中Cr、BUN和CRP水平均明显升高(P < 0.01);与IR组比较,I-postC组大鼠血浆中Cr、BUN和CRP水平均降低(P < 0.01)。见表 1。
| [n=10,x± s,λB/(U·L-1)] | |||
| Group | Cr | BUN | CRP |
| Control | 21.55±3.90 | 5.06±1.62 | 3.53±0.21 |
| IR | 58.93±6.76* | 17.09±2.06* | 6.05±0.80* |
| I-postC | 27.86±2.31*△ | 12.04±1.41*△ | 4.98±0.68*△ |
| *P<0.01 vs control group;△P<0.01 vs IR group. | |||
免疫组织化学染色,TUNEL阳性信号呈棕黄色。对照组大鼠肾组织中TUNEL阳性细胞即凋亡细胞较少;IR组大鼠肾组织可见较多TUNEL阳性细胞;I-postC组大鼠肾组织TUNEL阳性细胞较IR组明显减少。见图 1(插页三)。
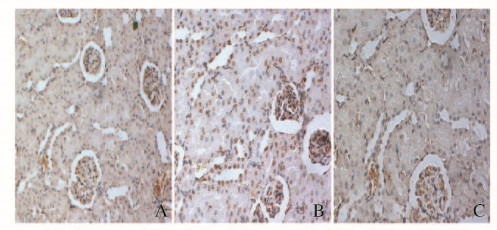
|
| A:Control group; B: IR group; C: I-postC group. 图 1 免疫组织化学法检测各组大鼠肾组织细胞凋亡(×200) Figure 1 Apoptosis of kidney tissue of rats in various groups detected by immunohi stochemistry(×200) |
|
|
激光共聚焦显微镜下观察,对照组大鼠肾组织中凋亡细胞罕见;IR组大鼠肾组织中可见较多TUNEL阳性信号,呈黄绿色或黄色荧光,位于胞核,呈小圆形、环行或颗粒状;I-postC组大鼠肾组织中TUNEL阳性细胞较IR组明显减少。见图 2(插页三)。
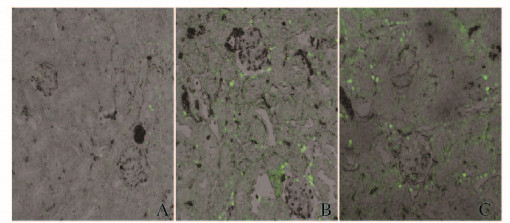
|
| A:Control group; B: IR group; C: I-postC group. 图 2 激光共聚焦显微镜下观察各组大鼠肾组织细胞凋亡(×200) Figure 2 Apoptosis of kidney tissue of rats in various groups observed under laser confocal microscope(×200) |
|
|
Bcl-2和Bax蛋白阳性表达主要位于肾小管上皮细胞胞浆内,呈棕黄色颗粒。对照组大鼠肾组织可见少量肾细胞胞浆呈浅棕黄色,即Bcl-2和Bax蛋白弱阳性表达;再灌注后二者表达均增多,且IR组较I-postC组大鼠肾组织中阳性表达的细胞数目多,着色深。见图 3(插页四)。
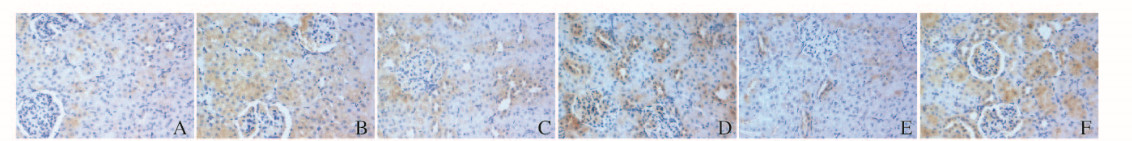
|
| A-C:Bcl-2 protein; D-F:Bax protein; A, D: Control group; B, E: IR group; C, F: I-postC group. 图 3 各组大鼠肾组织中Bcl-2和Bax蛋白表达(×200) Figure 3 Expressions of Bcl-2 and Bax protein in kidney tissue of rats in various groups(×200) |
|
|
与对照组比较,IR组和I-postC组大鼠肾组织中Bax和Bcl-2表达水平明显升高(P < 0.05或P < 0.01),但Bcl-2表达强度弱于Bax,Bcl-2/Bax比值降低(P < 0.05);与IR组比较,I-postC组大鼠肾组织中Bax表达水平降低(P < 0.05),Bcl-2表达水平升高(P < 0.01),Bcl-2/Bax比值升高(P < 0.05)。见表 2。
| (n=10, x± s) | |||
| Group | Bcl-2 | Bax | Bcl-2/Bax |
| Control | 0.15±0.01 | 0.13±0.02 | 1.15±0.18 |
| IR | 0.28±0.03* | 0.35±0.02** | 0.80±0.08* |
| I-postC | 0.36±0.02**△△ | 0.32±0.03**△ | 1.13±0.09*△ |
| *P < 0.05, **P < 0.01 vs control group; △P < 0.05, △△P < 0.01 vs IR group. | |||
透射电镜下观察,对照组大鼠肾组织细胞结构清晰完整。再灌注后,IR组大鼠肾组织中近曲小管上皮细胞核固缩,线粒体数量减少,部分线粒体嵴断裂或模糊,甚至空泡化;肾小球足突细胞足突排列不规则,部分突起融合;线粒体肿胀,空泡变性较明显,线粒体嵴断裂并减少,粗面内质网扩张。与IR组比较,I-postC组大鼠肾组织中肾小管上皮细胞和肾小球细胞损伤有一定程度改善。见图 4。

|
| A-C:Glomerulus; D-F:Renal tubules; A, D: Control group; B, E: IR group; C, F: I-postC group. 图 4 电镜下观察各组大鼠肾组织超微结构(bar=1 μm) Figure 4 Ultrastructures of kidney tissue of rats in various groups under electron microscope(bar=1 μm) |
|
|
LIR可经多途径触发全身组织细胞凋亡程序,死亡细胞数目的增多,可严重影响器官的功能[5]。在LIR致肾损伤的过程中,肾小管上皮细胞和肾小球毛细血管内皮细胞的过度凋亡是急性肾功能障碍的重要机制[6]。目前,缺血后处理加再灌注是防治缺血再灌注损伤的有效措施之一,具有可控性好、操作简单和无毒副作用等特点[4]。
近年来研究[7]显示:LIR激发的氧化应激、钙超载、炎症因子活化和能量代谢障碍等均会导致局部和远位组织细胞凋亡增多,和细胞坏死一起,参与全身性的再灌注损伤过程。CRP是一种非特异性炎性标志物,可在损伤和炎症急性期敏感表达,参与细胞凋亡的发生发展过程[8]。Bcl-2和Bax属于Bcl-2基因家族成员,在细胞凋亡的过程中,二者相互作用并抑制对方活性[9-10]。本研究结果显示:在远端肾小管,再灌注后Bcl-2高表达,IR组大鼠Bcl-2/Bax比值降低,而I-postC组大鼠Bcl-2/Bax比值有所升高。Bcl-2抑制细胞凋亡的作用与抗氧化、抑制Ca2+内流等因素有关[11],其通过抑制细胞信号传递而发挥抑制细胞凋亡、延长细胞寿命的作用。缺血后处理加再灌注可通过激发机体自身防护系统而抑制细胞凋亡的发生,如降低线粒体膜通透性、阻止促凋亡蛋白从线粒体向外释放和抑制Caspases的激活[12]。在细胞凋亡程序中,Caspases蛋白酶以前酶形式存在, 在上游分子的作用下序贯性激活,最终由Caspase-3在细胞凋亡执行阶段发挥作用,通过激活DNA酶,引起以DNA片断和细胞形态学改变为特征的细胞凋亡。缺血后处理加再灌注还可抑制Bax和Bak的细胞毒作用,阻止细胞的过度凋亡过程[13]。Wever等[14]研究也显示:短暂的后肢缺血可通过非腺苷依赖的机制减轻肾脏的损伤。本研究中,各组大鼠肾组织中细胞凋亡情况的形态学证据和血浆中Cr及BUN水平从功能学角度也支持缺血后处理加再灌注对肾脏具有保护作用。
综上所述,在血流再灌注之前进行缺血后处理,可抑制LIR过程中肾组织细胞的凋亡,进而减轻后继急性肾损伤的程度,本研究为临床全面防治缺血再灌注损伤提供了参考依据。
| [1] | Men X, Han S, Gao J, et al. Taurine protects against lung damage following limb ischemia reperfusion in the rat by attenuating endoplasmic reticulum stress-induced apoptosis[J]. Acta Orthop, 2010, 81(2): 263–267. DOI:10.3109/17453671003587085 |
| [2] | Zhao W, Gan X, Su G, et al. The interaction between oxidative stress and mast cell activation plays a role in acute lung injuries induced by intestinal ischemia-reperfusion[J]. J Surg Res, 2014, 187(2): 542–552. DOI:10.1016/j.jss.2013.10.033 |
| [3] | Kin H, Zhao ZQ, Sun HY, et al. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion[J]. Cardiovasc Res, 2004, 62(1): 74–85. DOI:10.1016/j.cardiores.2004.01.006 |
| [4] | Cao XL, Du J, Zhang Y, et al. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats[J]. Exp Brain Res, 2015, 233(10): 2753–2765. DOI:10.1007/s00221-015-4269-x |
| [5] | 赵利军, 李开济, 卢秋玲, 等. 缺血后处理对大鼠肢体缺血再灌注后肺损伤的保护作用及其机制[J]. 吉林大学学报:医学版, 2016, 42(2): 255–259. |
| [6] | Zambas NA, Karkos CD, Kambaroudis AG, et al. Protective effect of antithrombin Ⅲ against lung and myocardial injury in lower-limb ischemia-reperfusion syndrome[J]. Ann Vasc Surg, 2012, 26(4): 566–570. DOI:10.1016/j.avsg.2012.01.004 |
| [7] | Shevtsov MA, Nikolaev BP, Yakovleva LY, et al. Neurotherapeutic activity of the recombinant heat shock protein Hsp70 in a model of focal cerebral ischemia in rats[J]. Drug Des Devel Ther, 2014, 8: 639–650. |
| [8] | Mori S, Sawada T, Okada T, et al. Erythropoietin and its derivative protect the intestine from severe ischemia/reperfusion injury in the rat[J]. Surgery, 2008, 143(4): 556–565. DOI:10.1016/j.surg.2007.12.013 |
| [9] | Zhang Y, Leng YF, Xue X, et al. Effects of penehyclidine hydrochloride in small intestinal damage caused by limb ischemia-reperfusion[J]. World J Gastroenterol, 2011, 17(2): 254–259. DOI:10.3748/wjg.v17.i2.254 |
| [10] | Zhang J, Wu Y, Weng Z, et al. Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway[J]. Brain Res, 2014, 1582: 176–186. DOI:10.1016/j.brainres.2014.07.002 |
| [11] | ShinoharaG, MoritaK, NagahoriR, et al. Ischemic postconditioning promotes left ventricular functional recovery after cardioplegic arrest in an in vivo piglet model of global ischemia reperfusion injury on cardiopulmonary bypass[J]. J Thorac Cardiovasc Surg, 2011, 142(4): 926–932. DOI:10.1016/j.jtcvs.2011.01.028 |
| [12] | 李开济, 贺宝玲, 卢秋玲, 等. 缺血后处理减轻大鼠肢体缺血再灌注后肺损伤的实验研究[J]. 天津医药, 2016, 44(4): 453–456. |
| [13] | Pang L, Ma H, Cai Y, et al. Inhibition of COX-2/PGE2/EP4 signaling protects against post-hypoxic apoptosis In H9C2 cardiomyocytes[J]. FASEB, 2016, 30: 937–939. |
| [14] | Wever KE, Warlé MC, Wagener FA, et al. Remote ischemic preconditioning by brief hind limb ischemia protects against renal ischemia-reperfusion injury:the role of adenosine[J]. Nephrol Dial Transplant, 2011, 26(10): 3108–3117. DOI:10.1093/ndt/gfr103 |
 2017, Vol. 43
2017, Vol. 43


