扩展功能
文章信息
- 伊琳, 陈蓓蓓, 孙少伯, 杨陇, 纪禄风, 石向慧
- YI Lin, CHEN Beibei, SUN Shaobo, YANG Long, JI Lufeng, SHI Xianghui
- 吉林大学学报(医学版), 2016, 42(04): 671-675
- Journal of Jilin University (Medicine Edition), 2016, 42(04): 671-675
- 10.13481/j.1671-587x.20160407
-
文章历史
- 收稿日期: 2015-11-20
2. 甘肃中医药大学基础医学院 医用生物学教研室, 甘肃 兰州 730000;
3. 甘肃省通渭县人民医院外科, 甘肃 通渭743300
2. Department of Medical Biology, College of Basic Medical Sciences, Gansu University of Chinese Medicine, Lanzhou 730000, China;
3. Department of Surgery, Tongwei People's Hospital, Gamsu Province, Tongwei 743300, China
微小RNA(microRNAs,miRNAs)是由一段具有发夹环结构的长度为70~80个核苷酸的miRNA前体剪切后生成,且其在进化上具有高度的保守性,能够通过与靶mRNA特异性的碱基互补配对,引起靶mRNA降解或抑制其翻译,从而对基因进行转录后的表达调控[1]。近年来,miRNA在心脑血管疾病中的作用被日益重视,在左心室肥大、缺血性心脏病、冠心病、心律失常和高血压病等心血管(系统)疾病中可作为重要的生物标记物[2]。当归是传统中药,来源于伞形科(Umbelliferae)当归属(Angelica),研究[3]表明:以中药当归为主药的中药血灵具有降压作用,但关于当归中的主要有效成分当归挥发油对血压的调节作用及其对心肌组织中miRNA表达谱影响的研究较少。本研究选用自发性高血压大鼠(spontaneously hypertensive rats,SHRs)为研究对象,探讨当归挥发油对SHRs心肌组织中miRNA表达谱的影响,为探讨当归在高血压发生发展过程中的作用机制和靶点提供依据。
1 材料与方法 1.1 实验动物8周龄雄性SHRs及Wistar大鼠,体质量180~220 g,SPF级。SHRs动物合格证号为11400700069126;Wistar大鼠动物合格证号为62001201604070161,许可证号为SCXK(甘)2011-0001。SHRs随机分为模型组、卡托普利组和当归组,Wistar 大鼠作为对照组,每组 8只。实验程序和方法得到甘肃中医药大学实验动物伦理委员会批准。
1.2 药物、试剂和仪器超临界CO2萃取当归挥发油(藁本内酯>70%),甘肃岷县康达药业开发有限责任公司提供;卡托普利,山西津华晖星制药有限公司提供; Power Lab信号采集分析系统(澳大利亚AD Instruments公司,型号:ML845 Power Lab 4/25)。
1.3 给药方式及血压测量采用 Power Lab信号采集分析系统无创性套尾法测定大鼠清醒状态下尾动脉收缩压。测定血压前将大鼠鼠尾放入(37±1)℃温水内预热,安装尾套,同时使大鼠尾动脉与Power Lab信号采集分析系统的脉搏传感器紧密接触。待动物安静并出现稳定的脉搏波时即可开始测定血压。正式用药前,适应性测量血压1周。当归挥发油用吐温-80助溶,当归组大鼠给予100 mg·kg-1·d-1当归挥发油灌胃,卡托普利组大鼠给予12.5 mg·kg-1·d-1卡托普利灌胃,模型组和对照组大鼠给予与当归组同体积溶媒灌胃,每天1次,持续4周,每周测量大鼠血压。
1.4 样本采集和miRNA表达谱芯片检测给药4周后,大鼠禁食 12 h,称体质量,水合氯醛麻醉后将大鼠颈椎脱臼处死,开胸取出心肌组织。DEPC 水冲洗后,取心肌组织约100 mg,立即置于液氮保存,送至博奥生物有限公司进行RNA提取及表达谱的测定。采用Affymetrix miRNA 4.0 芯片杂交技术进行miRNA表达谱的检测分析。 采用信号比值(Ratio)法进行差异基因筛选,Ratio>2.0表示基因表达上调,Ratio<0.5表示基因表达下调。样品制备方法编号为AG-SP-RI21-01-2012 ,样品质量控制方法及标准编号为 AG-AO-QC01-01-2012。使用miRDB、miRWalk、PITA和Targetscan 等预测网站对大鼠进行靶基因的分析预测,取已验证的靶基因进行京东基因与基因组百科全书(KEGG)富集分析。
1.5 统计学分析
采用 SPSS 21.0统计软件包进行统计学处理。大鼠尾动脉收缩压以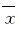 ±s表示,组间比较用单因素方差分析。以P<0.05为差异有统计学意义。
±s表示,组间比较用单因素方差分析。以P<0.05为差异有统计学意义。
给药前模型组、卡托普利组和当归组大鼠收缩压明显高于对照组 (P<0.05); 连续给药1~4周后,卡托普利组和当归组大鼠收缩压明显低于模型组(P<0.05)。见表1。
(n=8,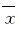 ±s,P/mmHg) ±s,P/mmHg) | |||||
| Group | Systolic blood prossure | ||||
| Before treatment | 1 week | 2 weeks | 3 weeks | 4 weeks | |
| Control | 139±2 | 140±4 | 141±4 | 141±3 | 140±3 |
| Model | 188±6 * | 194±9 * | 199±4 * | 201±3 * | 199±3 * |
| Captopril | 187±4 * △ | 173±2 * △ | 164±1 * △ | 145±3 * △ | 129±2 * △ |
| Angelica | 187±8 * △ | 182±8 * △ | 172±2 * △ | 169±2 * △ | 155±9 * △ |
| * P<0.05 compared with control group; △ P<0.05 compared with model group. | |||||
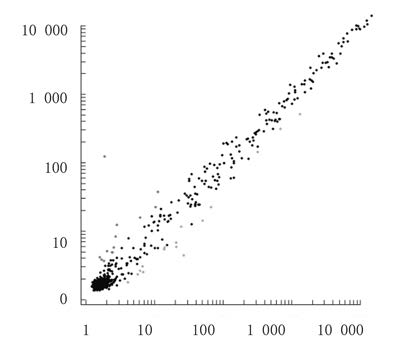
采用信号值比值法进行差异基因筛选。与模型组比较,当归组大鼠呈差异性表达的 miRNA 共29个,其中表达上调的miRNA 13个(Ratio>2.0,P<0.05),表达下调的miRNA 16个(Ratio<0.5,P<0.05)。见图1和表2、3。
|
| 图1 当归组大鼠差异表达的miRNA双对数散点图 Fig.1 Log-Log scatter plot of differential expressions of miRNAs of rats in angelica group |
| Probe Set ID | MiRNA | Ratio |
| 20524314 | miR-3068 | 3.4268 |
| 20513721 | miR-362 | 2.1368 |
| 20500944 | miR-326 | 4.4115 |
| 20501032 | miR-101b | 3.0411 |
| 20501329 | let-7i | 2.0592 |
| 20501350 | miR-19a | 2.5352 |
| 20501420 | miR-101a | 3.5109 |
| 20501428 | miR-122 | 87.8397 |
| 20501501 | miR-187 | 2.0357 |
| 20501522 | miR-200b | 2.2357 |
| 20501539 | miR-181a | 2.0829 |
| 20504490 | miR-499 | 2.3822 |
| 20504491 | miR-664 | 2.931 0 |
| Probe Set ID | MiRNA | Ratio |
| 20500950 | miR-328a | 0.4838 |
| 20500958 | miR-330 | 0.4567 |
| 20500993 | miR-342 | 0.3849 |
| 20501433 | miR-125a | 0.3513 |
| 20501440 | miR-127 | 0.1661 |
| 20501461 | miR-138 | 0.2770 |
| 20501487 | miR-181c | 0.4951 |
| 20501536 | miR-211 | 0.4334 |
| 20501566 | miR-298 | 0.3686 |
| 20504251 | miR-487b | 0.4320 |
| 20506496 | miR-881 | 0.3681 |
| 20506533 | miR-455 | 0.4722 |
| 20506546 | miR-671 | 0.4617 |
| 20506553 | miR-708 | 0.4126 |
| 20513755 | miR-547 | 0.3894 |
| 20524321 | miR-3473 | 0.3831 |
当归组大鼠表达上调的miRNA13个,已验证靶基因237个;当归组大鼠表达下调的miRNA16个,已验证靶基因111个。KEGG富集分析差异表达的miRNA的靶基因结果:miR-19a、 let-7i和miR-181c与胰岛素信号通路有关联(P<0.05),miR-122、miR-181a、miR-200b、miR-181c、let-7i和miR-19a与细胞凋亡有关联(P<0.05),let-7i、miR-181a和miR -455与血管皮内生长因子(VEGF)信号通路调控有关联(P<0.05)。见表4。
| Pathway | miRNA | Target gene |
| Insulin signaling pathway | rno-miR-19a | Socs1,Kras,Akt1 |
| Insulin signaling pathway | rno-let-7i | Socs1,Kras,Akt1,Socs3,Grb2,Mapk1,Mapk3,Ppargcla |
| Insulin signaling pathway | rno-miR-181c | Socs1,Kras,Akt1 |
| Apoptosis | rno-miR-122 | I11a,Tnf,Tp53,Bcl2,Fas |
| Apoptosis | rno-miR-181a | Casp3,Tnf,Tp53,Bcl2 |
| Apoptosis | rno-miR-200b | Akt1,Tp53,Bcl2 |
| Apoptosis | rno-miR-181c | Akt1,Tp53,Bcl2 |
| Apoptosis | rno-let-7i | Tnf,Tp53,Bcl2,Fas,Birc2,BCLX RAT,Akt1,Rela |
| Apoptosis | rno-miR-19a | Akt1,Tp53,Bcl2 |
| VEGF signaling pathway | rno-let-7i | Mapk1.Mapk3,Kras,Akt1 |
| VEGF signaling pathway | rno-miR-181a | Mapk14,Mapk3,Prkcc,Kras |
| VEGF signaling pathway | rno-miR-455 | Mapk14,Mapk3 |
高血压作为心血管事件的重要危险因素,常引起严重的心、脑和肾等器官的损伤。以往研究 [4, 5]显示:当归可以对SHRs脑组织基因表达谱产生影响且与信号转导相关基因有关。作为表观遗传学的重要方面,miRNA在高血压发生发展 过程中起重要作用。本研究采用Affymetrix miRNA 4.0芯片检测分析miRNA表达谱结果显示:与模型组比较,当归组大鼠差异性表达的 miRNA 共29个,其中表达上调13个,表达下调16个;对miRNA的靶基因进行KEGG富集分析结果显示:miR-19a、 let-7i和miR-181c与胰岛素信号通路有关联,let-7i 、miR-181a和miR-455与VEGF信号通路调控有关联,miR-122、miR-181a、miR-200b、miR-181c、let-7i和miR-19a与细胞凋亡有关联。胰岛素抵抗是2型糖尿病和高血压的共同病理生理基础。人血管紧张素1-7(Ang 1-7) 可能通过 Mas /PI3K /AKT 通路促进心房钠尿肽分泌,保护心脏,通过Mas激活 AKT,调控胰岛素信号通路,增强胰岛素敏感性[6]。ACE2/Ang1-7/Mas受体轴的激活可阻滞炎性因子的产生,降低氧化应激,从而提高胰岛素敏感性及分泌[7];另一方面,激活的血管紧张素Ⅱ1型受体,能够促进胰岛素抵抗的发展,降低血管的胰岛素敏感性,导致内皮释放NO量减少,使血压升高[8]。研究[9]表明:miR-122通过基因多态性(SNP)变化引起SLC7A1基因表达下调,使 NO水平降低,导致内皮功能紊乱,进而影响高血压的发生。本研究结果显示:miR-19a、 let-7i和miR-181c在当归组大鼠中表达明显上调,且均与胰岛素信号通路有关联,当归是否能通过miR-19a、 let-7i和miR-181c作用于胰岛素信号通路改善内皮功能进而发挥降压作用有待进一步验证。 VEGF是内皮细胞发育中重要的细胞因子。VEGF 受体(VEGFR)主要包括VEGFR1、VEGFR2和VEGFR3[10],VEGF 促进血管生长的途径与 VEGFR2 关系最为密切,且VEGF 和VEGFR2结合主要通过 PI3K下游 Akt信号分子传导信号,进一步刺激内皮型一氧化氮合酶 (eNOS) 基因的表达,从而引起血管内皮修复和抗凋亡作用[11]。let-7家族中miRNA,miR-21、 miR-126、miR-221和miR-222在内皮细胞中高表达,Dicer沉默后,let-7家族中miRNA表达减少,且血管出芽形成减少,let-7家族中的let-7f 和miR-27b作用于抗血管生成基因可促进新生血管形成[12]。内皮细胞功能紊乱对高血压的发生发展具有重要作用,两者相互影响[13]。本研究中let-7i 、miR-181a和miR-455与VEGF信号通路调控有关联,当归是否可能通过let-7i 、miR-181a和miR-455VEGF信号通路,改善内皮功能来发挥降压作用值得进一步探讨。
研究[14, 15]表明:在高血压的发展进程中,心肌细胞增生与凋亡调节失衡会导致心肌肥厚,且肾素-血管紧张素系统(RAS) 的激活可诱导心肌细胞和心肌间质细胞凋亡,心肌纤维化与凋亡明显增加。心肌肥大与心肌纤维化相互作用影响[16],且均与凋亡有密切关联。miR-122、miR-181a、miR-200b、miR-181c、let-7i和miR-19a与凋亡信号通路有关联。本研究中当归组大鼠let-7i、miR-101b 和miR-328也呈差异表达。let-7i能明显抑制血管紧张素Ⅱ诱导的心肌纤维化[17],同时经血管紧张素Ⅱ干预的心肌成纤维细胞中miR-101a/b表达水平降低,miR-101a/b可通过抑制大鼠心肌成纤维细胞的增殖及胶原蛋白产生来抑制心肌间质纤维化[18]。由于miR-101作用于其靶基因可抑制心肌肥厚,故其已成为治疗心肌肥厚的潜在靶标[19]。miR-328可靶向作用于 肌浆/内质网Ca2+-ATP酶2a( SERCA2a),促进心肌肥厚 [20]。本研究中,SHRs心肌组织中let-7i及miR-101b表达上调,miR-328表达下调,分析其原因可能为:当归挥发油作用于let-7i、miR-101b和miR-328,虽未对高血压的发生有明显影响,但可能通过抑制心肌纤维化或心肌肥大来保护心肌细胞。
综上所述,当归挥发油可能作用于胰岛素相关信号通路及VEGF信号通路影响内皮功能来发挥血压调节作用,同时可能通过调节细胞凋亡信号通路抑制心肌纤维化或心肌肥大来保护心肌细胞。在后续的研究中,将采用实时定量RT-PCR对差异表达的miRNA进行验证,探索miRNA作用路径及相关调控机制。
| [1] | Schmiedel JM,Klemm SL,Zheng Y,et al. Gene expression. MicroRNA control of protein expression noise[J] . Science,2015,348(6230):128-132. |
| [2] | Romaine SP,Tomaszewski M,Condorelli G,et al. MicroRNAs in cardiovascular disease:an introduction for clinicians[J]. Heart,2015,101(12):921-928. |
| [3] | 程 远,吕圭源,陈素红. 浅谈当归制剂降血压的临床 应用[J] . 亚太传统医药,2011,7(4):138-139. |
| [4] | 伊 琳,赵 昕,李 屹. 当归对原发性高血压大鼠脑组织基因表达谱的影响[J]. 中国现代医学杂志,2014, 24(11):24-27. |
| [5] | 伊 琳,赵 昕,李 屹. 当归对自发性高血压大鼠脑组织 Tnfaip812、Ahsg 及 Tlr3 基因表达的影响[J]. 中国动脉硬化杂志,2013,21(10):891-893. |
| [6] | 崔立建,刘瑞霞,王 艳,等. ACE2-Ang (1-7) -Mas 轴的生物学功能及其下游信号通路[J]. 基础医学与临床,2014,34(12):1718-1722. |
| [7] | Favre GA,Esnault VLM,van Obberghen E. Modulation of glucose metabolism by the rennin-angiotensin-aldoterone system[J]. Am J Physiol-Endocrinol Matabol,2015,308(6):E435-E449. |
| [8] | Manrique C,Lastra G,Sowers JR. New insights into insulin action and resistance in the vasculature[J]. Ann N Y Acad Sci,2014,1311(1):138-150. |
| [9] | Yang Z,Kaye DM. Mechanistic insights into the link between a polymorphism of the 3' UTR of the SLC7A1 gene and hypertension[J]. Hum Mutat,2009,30(3):328-333. |
| [10] | Shibuya M . Vascular endothelial growth factor and its receptor system:physiological functions in angiogenesis and pathological roles in various diseases[J]. J Biochem,2013,153(1):13-19. |
| [11] | Gerber HP ,McMurtrey A,Kowalski J,et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway:requirement for Flk-1/KDR activation[J] . J Biol Chem, 1998,273(46):30336-30343. |
| [12] | Kuehbacher A,Urbich C,Zeiher AM,et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis[J]. Circ Res, 2007,101(1):59-68. |
| [13] | 钟南山,陆再英.内科学[M].北京:人民卫生出版社,2008:256-276. |
| [14] | Moreau P ,Tea BS,Dam TV,et al. Altered balance between cell replication and apoptosis in hearts and kidneys of newborn SHR[J]. Hypertension,1997,30(3Pt2):720-724. |
| [15] | Kobori H ,Ichihara A,Miyashita Y,et al. Local renin-angiotensin system contributes to hyperthyroidism-induced cardiac hypertrophy[J]. J Endocrinol,1999,160(1):43-47. |
| [16] | Nadruz W. Myocardial remodeling in hypertension[J]. J Hum Hypertens,2015,29(1):1-6. |
| [17] | Wang X ,Wang HX,Li YL,et al. MicroRNA Let-7i negatively regulates cardiac inflammation and fibrosis[J]. Hypertension,2015,66(4):776-785. |
| [18] | Pan Z ,Sun X,Shan H,et al. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-β1 pathway[J]. Circulation,2012,126(7):840-850. |
| [19] | Wei L,Yuan M,Zhou R,et al. MicroRNA-101 inhibits rat cardiac hypertrophy by rargeting Rab1a [J]. J Cardiovasc Pharmacol,2015,65(4):357-363. |
| [20] | Yin K,Zhao L,Feng D,et al. Resveratrol Attenuated Low Ambient Temperature-Induced Myocardial Hypertrophy via Inhibiting Cardiomyocyte Apoptosis[J]. Cell Physiol Biochem,2015,35(6):2451-2462. |
 2016, Vol. 42
2016, Vol. 42


