扩展功能
文章信息
- 吴嫣然, 杨陆一, 刘军, 殷红, 牟胤赫, 夏小雪, 滕蓉
- WU Yanran, YANG Luyi, LIU Jun, YIN Hong, MOU Yinhe, XIA Xiaoxue, TENG Rong
- 类金刚石涂层与未涂层镍钛丝在人工唾液中耐腐蚀性能的比较
- Comparison of corrosion resistance abilities between diamond-like carbon coated and uncoated nickel titanium wire in artificial saliva
- 吉林大学学报(医学版), 2016, 42(03): 517-522
- Journal of Jilin University (Medicine Edition), 2016, 42(03): 517-522
- 10.13481/j.1671-587x.20160319
-
文章历史
- 收稿日期: 2016-03-20
2. 吉林大学超硬材料国家重点实验室, 吉林 长春 130021
2. State Key Laboratory of Superhard Materials, Jilin University, Changchun 130021, China
镍钛弓丝(镍钛丝)以其独特的超弹性、形态记忆功能和良好的生物相容性在正畸治疗中应用了几十年[1]。然而,镍钛合金在口腔中能否长期存在仍有争议,因为在不同pH值和氟离子(F-)浓度的口腔唾液中,镍钛合金均可通过离子析出或者电化学腐蚀作用使材料降解[2, 3]。其腐蚀产物会导致诸多健康问题,例如过敏反应、毒性反应和致癌作用[4]。此外,镍钛丝较其他正畸弓丝有较高的摩擦系数[5],腐蚀后其表面粗糙程度相对增大,不利于牙齿的轻力移动。为改善正畸弓丝的长期生物相容性和摩擦学性能,已有许多不同表面处理方法应用到正畸弓丝上[6, 7, 8, 9]。类金刚石涂层(diamond-like carbon,DLC)以其优越的性能,例如超硬、低摩擦、化学惰性和耐腐蚀等[10],不仅广泛应用于工业产品,在生物医学领域也得到越来越多的关注。1993年,Kusy等[11]将DLC应用于正畸领域,证实该涂层可以较大程度降低多晶氧化铝/β钛丝间的摩擦力。近几年,人们利用化学气相沉积或者物理气相沉积等方法将DLC应用到托槽和正畸弓丝等材料上,研究其生物安全性以及摩擦学性能等[12, 13, 14],但是对于DLC涂层镍钛丝在不同pH值和F-浓度的人工唾液中的电化学腐蚀性能的相关研究尚未见报道。本研究利用射频磁控溅射技术在镍钛丝上制备DLC涂层,比较其与未涂层镍钛丝在不同pH值和F-浓度的人工唾液中的耐腐蚀性能。
1 材料与方法 1.1 主要试剂和仪器0.018*0.025镍钛丝(日本TOMY公司 ),人工唾液各成分试剂(中国实验室试剂耗材采购网公司),去离子水、无水乙醇和丙酮(北京化工厂)。射频磁控溅射器(中国科学院沈阳科学仪器股份有限公司),CHI920 C型电化学工作站(上海辰华公司),超声清洗机(CLEAN-01 型,宁波蓝野医疗器械有限公司),pH 计(美国Thormo 公司),电子天平(MP500Z 型,上海舜宇恒平科学仪器有限公司 ),场发射扫描电子显微镜电镜(SEM,XL30 ESEM FEG,美国FEI公司),激光拉曼光谱(H30434型,英国Renishaw公司)
1.2 人工唾液的配制和分组采用 Fusayama-Meyer 型人工唾液配方,电子天平分别称出0.4 g KCl、0.4 g NaCl、0.906 g CaCl2·2H2O、0.696 g NaH2PO4·2H2O、0.005 g Na2S·9H2O、1.00 g 尿素,用去离子水配至 1 000 mL,以NaOH和HCL调整pH值,NaF调整F-的浓度。将人工唾液分为pH7+0%F-组、pH7+0.2% F-组、pH7+0.5% F-组和pH5+0.5% F-组。
1.3 样品的制备和分组截取0.018*0.025 TOMY镍钛丝末端较平直部分,长约20 mm,逐级经600#和1 200#砂纸打磨,丙酮、无水乙醇、去离子水依次超声清洗5 min,干燥备用。从中随机选取24段镍钛丝在其较宽的2个面上制备DLC涂层(该工作由吉林大学超硬材料国家重点实验室完成)作为实验组,射频磁控溅射涂层的工艺参数为偏压-100 V,射频电源功率120 W,气体选用氩气(Ar),流量为20 SCCM,工作气压为1.0 Pa,基体温度为125℃,沉积时间为60 min。膜厚度为±1 μm。随机选取24段未经涂层处理的镍钛丝作为对照组。所有实验用样品的一端以自凝塑料包埋连在导线上,通过计算弓丝长度,使每个样品的暴露面积相等,同时较窄的2个面涂布抗酸指甲油。实验组和对照组镍钛丝分别随机浸泡在4组配好的人工唾液中,每组6根。样品在新鲜配制的人工唾液中浸泡24 h。
1.4 2组镍钛丝自腐蚀电位(Ecorr)和腐蚀电流密度(Icorr)的测定采用CHI920 C型电化学工作站和Origin软件绘制动电位极化(Tafel)曲线。所用介质为室温下的人工唾液,研究电极为待测样品,参比电极为饱和甘汞电极,辅助电极为铂片。先测量研究电极的开路电位(OCP),待OCP稳定后(上下波动不超过5 mV)进行电位Tafel扫描并绘制Tafel曲线,此时比较稳定的开路电位电压值即为Ecorr,描绘Tafel曲线时选用的极化电位范围为开路电位上下200 mV,扫描速度设定为5 mV·s-1,找出Tafel曲线阳极和阴极的线性部分,其切线交点对应的电流值即为腐蚀电流,腐蚀电流与样品暴露表面面积的比值即为Icorr。
1.5 SEM观察各组镍钛丝表面形态学从实验组和对照组随机选取3根样品,采用SEM观察2组镍钛丝经人工唾液腐蚀后表面形态学,并与腐蚀前的表面形态学进行对比。
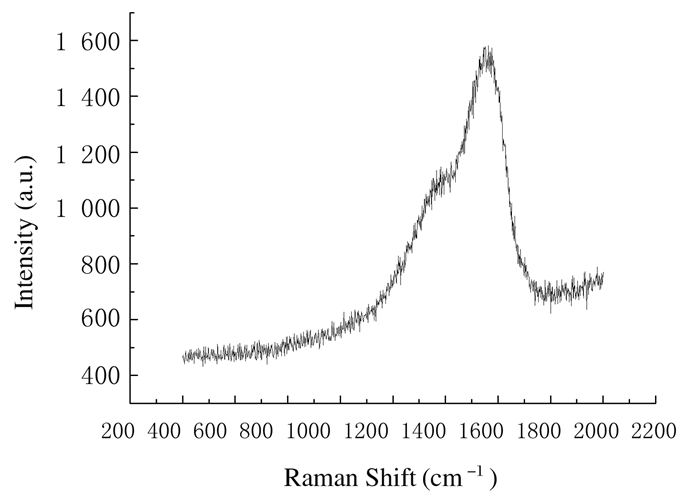
1.6 统计学分析采用SPSS 17.0统计软件对数据进行统计学分析。各组镍钛丝的Ecorr和Icorr均以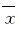 ± s表示。2组镍钛丝在不同组人工唾液中的Ecorr和Icorr比较采用单因素方差分析;不同种镍钛丝在同组人工唾液中的Ecorr和Icorr比较采用t检验。以α=0.05为检验水准。
± s表示。2组镍钛丝在不同组人工唾液中的Ecorr和Icorr比较采用单因素方差分析;不同种镍钛丝在同组人工唾液中的Ecorr和Icorr比较采用t检验。以α=0.05为检验水准。
在1 350 cm-1和1 520 cm-1处分别出现2个相对宽化的波峰(图 1),而出现明显变宽的D峰(约1 358 cm-1)和G峰 (约1 582 cm-1)是DLC结构的典型特征之一,证明在镍钛丝上覆盖的为DLC涂层。
|
| 图 1 DLC镍钛丝的拉曼光谱图 Fig.1 Raman spectra of DLC-NiTi wires |
随着F-浓度增加,对照组镍钛丝在4组人工唾液中的Ecorr逐渐降低(P < 0.05),Icorr呈升高趋势;与pH 7+0.5% F-组比较,对照组镍钛丝在pH5+0.5% F-组人工唾液中Ecorr降低(P < 0.05),Icorr升高(P < 0.05)。随着F-浓度增加,实验组镍钛丝在4组人工唾液中的Ecorr逐渐降低(P < 0.05),Icorr呈升高趋势;与pH7+0%F-组比较,实验组镍钛丝在pH7+0.2% F-组人工唾液中的Icorr升高(P < 0.05);与pH7+0.5% F-组比较,实验组镍钛丝在pH5+0.5% F-组人工唾液中的Ecorr降低(P < 0.05),Icorr升高(P < 0.05)。
实验组和对照组镍钛丝经4组人工唾液腐蚀后比较,pH7+0.2% F- 、pH7+0.5% F-、pH5+0.5% F-组人工唾液中,实验组镍钛丝的Ecorr均高于对照组(P < 0.05);实验组镍钛丝在pH7+0% F-组人工唾液中的Ecorr也高于对照组,但组间比较差异无统计学意义(P>0.05)。实验组镍钛丝在4组人工唾液中的Icorr均低于对照组(P < 0.05)。
(n=6,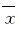 ± s
) ± s
) |
||||
| Group | Ecorr(U/mV) | Icorr(nA·cm-2) | ||
| Control | Experiment | Control | Experiment | |
| *P < 0.05 vs control group; △P < 0.05 vs pH7+0% F- group;#P < 0.05 vs pH7+0.2% F- group; ▲ P < 0.05 vs pH7+0.5% F-group. | ||||
| pH7+0%F- | -147.82±8.52 | -143.09±5.54 | 85.23±4.91 | 74.28±2.88* |
| pH7+0.2%F- | -206.81±4.53△ | -162.49±4.50*△ | 87.69±1.92 | 82.79±2.29*△ |
| pH7+0.5%F- | -308.92±11.82△# | -176.48±11.50*△# | 90.09±3.45 | 78.43±5.11* |
| pH5+0.5%F- | -325.47±4.50△#▲ | -263.95±4.56*△#▲ | 104.72±1.45△#▲ | 100.52±1.01*△#▲ |
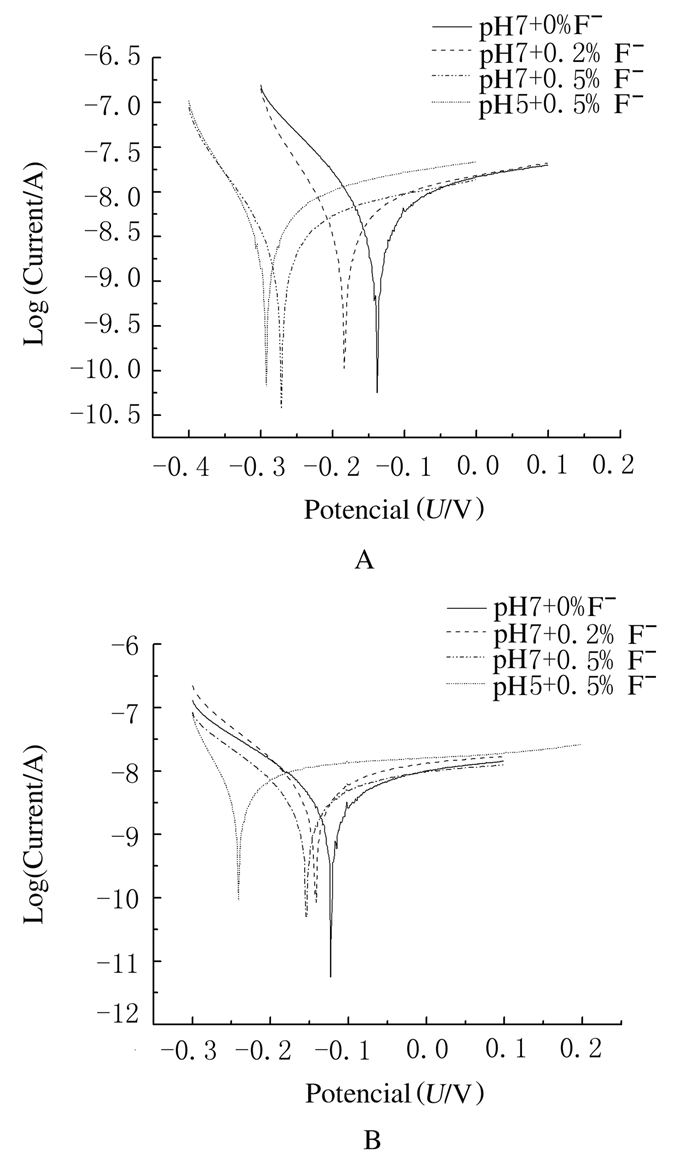
对照组镍钛丝在4组人工唾液中的Tafel曲线显示:Tafel曲线随着F-浓度增加和pH值降低依次向左偏移,与pH 7+0.5% F-组比较,pH5+0.5% F-组的Tafel曲线向上偏移(图 2A)。实验组镍钛丝在4组人工唾液中的Tafel曲线显示:Tafle曲线亦随着F-浓度增加和pH值降低依次向左偏移。pH5+0.5% F-组向左、向上偏移较多(图 2B)。对照组的4条Tafel曲线整体比实验组位置偏左。
|
| 图 2 2组镍钛丝在4组人工唾液中的Tafel曲线 Fig.2 Tafel curves of NiTi wires in two groups in four different kinds of artificial saliva A:Control group; B:Experiment group. |
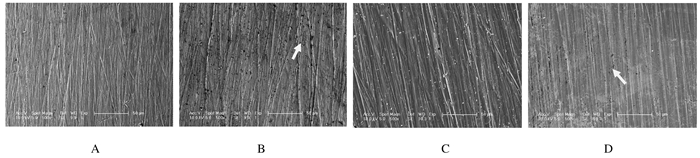
与腐蚀前比较,对照组镍钛丝经人工唾液腐蚀后可见多处散在凹坑,即点腐蚀;与腐蚀前比较,实验组镍钛丝经腐蚀后可见局部少量点腐蚀。见图 3。
|
| 图 3 SEM下观察人工唾液腐蚀前后2组镍钛丝表面形态表现(bar=50μm) Fig.3 Morphology of surface of NiTi wires before and after corrision with artificial saliva in two groups observed by SEM(bar=50 μm) A,B: Control group;C,D:Experiment group; A,C: Before corrision;B,D:After corrision. |
DLC涂层的制备方法常规分为2大类:一类是包括离子束沉积、溅射沉积和激光等离子沉积等方法的物理气相沉积;另一类是包括等离子体增强的化学气相沉积。本实验采用射频磁控溅射方法,其优点是低温条件下在镍钛丝上制备DLC涂层,可提高镍钛丝的耐腐蚀性能。金属腐蚀按腐蚀机制可分为电化学腐蚀和非电化学腐蚀2类。口腔环境中的金属腐蚀主要是电化学腐蚀[15],电化学极化测试也是评价医用金属材料抗腐蚀性能的一种有效方法[16],其优点为简便易行和精准度高。本研究采用电化学工作站经典三电极体系测量动电位极化曲线,其测定原理为控制电势电位以较慢的速度连续地改变(扫描),并测量对应电位下的瞬时电流值,以瞬时电流值与对应的电极电势绘图,获得整个极化曲线,即测定研究电极在连续变化电位下的半对数电流值,并在坐标轴上绘成的曲线。
Ecorr指在腐蚀体系中无外加电压时测得的稳定状态的开路电位(即稳定电位),能反映腐蚀倾向的大小,即Ecorr越低(负值越大),金属的腐蚀倾向越大;反之,腐蚀倾向越小。虽然利用Ecorr 值可以定性比较合金腐蚀的倾向,但其不能反映合金的实际腐蚀速率,而Icorr是评价合金腐蚀速率的重要参数,依赖于 Tafel外推法原理获得,首先从 Tafel 区呈线性关系的阴、阳极曲线做切线所得交点对应的纵坐标为腐蚀电流,再与暴露表面面积作比即为Icorr。随着F-浓度增大,对照组镍钛丝在4组人工唾液中的Ecorr逐渐降低,即腐蚀倾向逐渐增大;Icorr逐渐升高,即腐蚀速率呈加快趋势;Tafel曲线图可见:随着F-浓度的增大和pH值的降低,曲线依次向左偏移,说明F-的存在及浓度的增大降低了镍钛丝的耐腐蚀性能,这与马长柏等[17]的研究结果一致。pH5+0.5% F-组镍钛丝的Tafel曲线向上偏移表明:酸和F-共同作用使镍钛丝腐蚀倾向增大,腐蚀速率加快,这是因为氟化氢极易与镍钛表面的氧化膜TiO2发生一系列的反应,加速氧化膜的破坏[18]。随着F-浓度增大,实验组镍钛丝在4组人工唾液中的Ecorr降低,即腐蚀倾向逐渐增大,说明F-的存在及浓度的增大也影响了DLC镍钛丝的耐腐蚀性;酸和F-共同作用使DLC镍钛丝腐蚀倾向增大,腐蚀速率加快,具体原因和反应机制有待进一步研究,该趋势也有可能是局部DLC崩解,暴露内部镍钛丝表面,使得实验组DLC镍钛丝在酸性含F-人工唾液中的腐蚀规律与对照组相似。本研究结果显示:DLC涂层一定程度提高了镍钛丝在酸性及含F-人工唾液中的耐腐蚀性能。该结果与Huang等[1]研究结论一致(其研究结果显示:DLC涂层的镍钛弓丝表面粗糙度较未涂层镍钛弓丝减轻91.3%),反映出DLC涂层的保护作用。
对DLC涂层的表面形态学进行观察,除可借助原子力显微镜(AFM)或者3D激光显微镜外,也可借助SEM进行定性观察。本研究采用SEM观察镍钛丝在酸性含F-人工唾液中发生电化学腐蚀前后的表面形态学。在50μm视野下观察到对照组镍钛丝经腐蚀后表面有多处散在的点腐蚀,其原因为F-等阴性因子可以吸附在镍钛丝表面形成的TiO2钝化膜上,发生化学反应使氧原子排挤出去,氧化膜被破坏。此外,合金表面钝化膜成分除了TiO2还有少量碳元素,这些碳元素也会使钝化膜相对不稳定,这也是发生点腐蚀的原因之一。而实验组DLC镍钛丝经人工唾液腐蚀后可以观察到相对少量的点腐蚀,提示DLC可起到一定的保护作用。但是本文作者发现:个别DLC镍钛丝的点腐蚀聚成小段线条,这可能是由于DLC涂层表面局部出现微小裂痕,暴露内部镍钛丝发生腐蚀所致。
本研究结果显示:DLC对镍钛丝的电化学腐蚀有保护作用,但F-浓度增加及pH值降低均会影响其抗腐蚀性。这为探究DLC在正畸领域的应用提供了参考,也为镍钛丝表面的改性奠定了基础,但DLC与镍钛合金的结合强度及其在酸性含F-人工唾液中的反应机制有待于进一步研究。
| [1] | Huang SY, Huang JJ, Kang T,et al. Coating NiTi archwires with diamond-like carbon films: reducing fluoride-induced corrosion and improving frictional properties [J].J Mater Sci: Mater Med, 2013,24(10):2287-2292. |
| [2] | Huang H, Chiu YH, Lee TH, et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities [J]. Biomaterials, 2003,24(20): 3585-3592. |
| [3] | Li XJ, Wang JQ, Han EH, et al.Influence of fluoride and chloride on corrosion behavior of NiTi orthodontic wires [J]. Acta Biomateria, 2007,3(5):807-815. |
| [4] | Genelhu MC, Marigo M, Alves-Oliveira LF, et al. Characterization of nickel-induced allergic contact stomatitis associated with fixed orthodontic appliances [J]. Am J Orthod Dentofacial Orthop, 2005(128): 378-381. |
| [5] | Prashant PS, Nandan H, Gopalakrishnan M. Friction in orthodontics [J]. J Pharm Bioallied Sci, 2015, 7(Suppl 2): 334-338. |
| [6] | Espinar E, Llamas JM, Michiardi A, et al. Reduction of Ni release and improvement of the friction behaviour of NiTi orthodontic archwires by oxidation treatments [J]. J Mater Sci Mater Med, 2011,22(5): 1119-1125. |
| [7] | Kobayashi S, Ohgoe Y, Ozeki K, et al. Dissolution effect and cytotoxicity of diamond-like carbon coatings on orthodontic archwires [J]. J Mater Sci Mater Med, 2007,18(12): 2263-2268. |
| [8] | Xu JL, Liu F, Wang FP, et al. Alumina coating formed on medical NiTi alloy by micro-arc oxidation [J]. Mater Lett, 2008,62(25): 4112-4114. |
| [9] | Sun T, Lee WC, Wang M. A comparative study of apatite coating and apatite/collagen composite coating fabricated on NiTi shape memory alloy through electrochemical deposition [J]. Mater Lett, 2011,65(17): 2575-2577. |
| [10] | Roy RK, Lee KR. Biomedical applications of diamond-like carbon coatings: a review[J]. J Biomed Mater Res B Appl Biomater, 2007,83(1):72-84. |
| [11] | Kusy RP, Keith O, Whitley JQ, et al. Coefficient of friction characterization of surface-modified polycrystalline alumina [J]. J Am Ceram Soc,1993, 76(2):336-342. |
| [12] | Kobayashi S, Ohgoe Y, Ozeki K, et al. Dissolution effect and cytotoxicity of diamond-like carbon coatings on orthodontic archwires [J].J Mater Sci: Mater Med, 2007,18(12):2263-2268. |
| [13] | Bentahar Z, Barquins M, Clin M, et al. Tribological performance of DLC-coated stainless steel, TMA and Cu-NiTi [J].Int Orthodont, 2008 (6 ): 335-342. |
| [14] | Muguruma T,Iijima M,Brantley WA,et al. Frictional and mechanical properties of diamond-like carbon-coated orthodontic brackets [J]. Eur J Orthodont, 2011,35(2):216-222. |
| [15] | Chaturvedi TP. An overview of the corrosion aspect of dental implants (titanium and its alloys)[J]. Indian J Dent Res, 2009, 20(1):91-98. |
| [16] | Wang Q, Zhang Y, Hao FY, et al. Comparative study of clinically used NiTi orthodontic wires[J].Int J Mod PhysB, 2010, 24 (30): 5929-5937. |
| [17] | 马长柏,刘新强,李金华. 氟对镍钛弓丝的腐蚀性研究[J].现代口腔医学杂志, 2009, 23(6):587-590. |
| [18] | Yokoyama K, Kaneko K, Moriyama K, et al. Hydrogen embrittlement of NiTi super elastic alloy in fluoride solution [J].J Biomed Mater ResA, 2003(65):182-187. |
 2016, Vol. 42
2016, Vol. 42


