扩展功能
文章信息
- 胡志宏, 赵大力, 谢忠伟, 王梦莹, 胡景坤, 龚守良, 刘扬, 齐亚莉
- HU Zhihong, ZHAO Dali, XIE Zhongwei, WANG Mengying, HU Jingkun, GONG Shouliang, LIU Yang, QI Yali
- pEgr1-TRAIL重组质粒联合电离辐射对乳腺癌MCF-7细胞死亡受体通路相关基因和蛋白表达的影响
- Effects of pEgr1-TRAIL recombinant plasmid combined with ionizing radiation on related gene and protein expressions of death receptor pathway in breast cancer MCF-7 cells
- 吉林大学学报(医学版), 2016, 42(03): 419-423
- Journal of Jilin University (Medicine Edition), 2016, 42(03): 419-423
- 10.13481/j.1671-587x.20160301
-
文章历史
- 收稿日期: 2016-01-23
- 网络出版时间: 2016-05-17 14:04:05
2. 吉林省吉林(市)出入境检验检疫局, 吉林 吉林 132012;
3. 吉林大学公共卫生学院卫生部放射生物学重点实验室, 吉林 长春 130021
2. Jilin Province (City) Entry and Exit Inspection and Quarantine Bureau, Jilin Province, Jilin 132012, China;
3. Key Laboratory of Radiobiology, Ministry of Health, School of Public Health, Jilin University, Changchun 130021, China
近年来,我国居民恶性肿瘤的发病率和死亡率呈升高态势,其中肺癌、乳腺癌、肠癌和胃癌等发病率和死亡率上升尤为明显。传统的肿瘤治疗方法为手术切除后给予放、化疗,但其副反应较严重,治疗效果不够理想[1, 2]。肿瘤放射基因治疗既能够减少正常组织的放射损伤,又能促进肿瘤细胞凋亡,目前已成为肿瘤治疗的热点[3]。肿瘤坏死因子相关凋亡诱导配体(tumor necrosis factor related apoptosisinducing ligand,TRAIL)是TNF超家族成员,其在诱导肿瘤细胞凋亡的同时不损伤正常细胞[4, 5, 6]。另外,TRAIL还能结合放疗诱导肿瘤细胞凋亡,发挥杀死肿瘤细胞的协同效应[7, 8]。由于TRAIL基因位于Egr-1启动子的下游,电离辐射可以诱导和激活Egr-1基因,调节下游TRAIL基因的表达,进而激活死亡受体通路,诱导肿瘤细胞凋亡[9, 10]。本研究采用pEgr1-TRAIL重组质粒转染人乳腺癌MCF-7细胞,并联合电离辐射,观察人乳腺癌MCF-7细胞中死亡受体通路基因和蛋白表达水平,为乳腺癌的治疗提供实验依据。
1 材料与方法 1.1 细胞、质粒、主要试剂和仪器人乳腺癌MCF-7细胞系由吉林大学卫生部放射生物学重点实验室保存。pEgr1-TRAIL重组质粒和脂质体由刘扬博士制备。使用含10%胎牛血清的高糖DMEM(Sigma公司,美国)培养,培养条件为37℃、5% CO2、100%饱和湿度的培养箱,人caspase-9、caspase-6和DR4抗体购自美国Santa Cruz公司,Real-time检测试剂盒购自美国Sigma公司,其他试剂为国产分析纯。X射线深部治疗机购自日本Philips公司。
1.2 实验分组和照射方法将细胞分为对照组、空质粒组、pEgr1-TRAIL质粒组、4.0 GyX射线组、空质粒+4.0 GyX射线组和pEgr1-TRAIL+4.0 GyX射线组。采用X射线深部治疗机进行照射,照射条件为电压180 kV,电流12 mA,滤板厚度为铜0.5 mm、铝1.0 mm,剂量率为0.387 Gy·min-1,靶皮距为60 cm。
1.3 细胞转染将各组细胞按每孔2.0×105个接种于24孔板,使用不含抗生素的DMEM培养液培养24 h,当细胞密度达到约80%时进行转染。转染体系中脂质体与质粒的比例为3:1。将重组质粒和脂质体采用不含抗生素和胎牛血清的DMEM培养液稀释,至终浓度分别为1和3 g/50 L,5 min内将2种液体混合,共计400 μL,常温反应30 min后,接种于培养板中,置于37℃、5% CO2的培养箱中培养8 h,更换含抗生素和胎牛血清的DMEM培养液继续培养48 h。
1.4 Real-time PCR法检测各组细胞中DR4、caspase-9和caspase-6 mRNA表达水平将各组细胞接种于6孔培养板,密度为每孔4×105个细胞,设平行6复孔。培养24 h后,给予4.0 Gy X射线照射,照射后4、8、12和24 h收集细胞,提取总RNA,按照Real-time检测试剂盒说明书操作,检测细胞中DR4、caspase-9和caspase-6mRNA表达水平。采用相对定量2-ΔΔCt法计算目的基因相对于内参基因表达的倍数,比较基因的表达差异。每一个样本的ΔCt=Ct(target gene)-Ct(GAPDH),而ΔΔCt=ΔCt(target gene)-ΔCt(calibrator)。
1.5 Western blotting法检测各组细胞中DR4、caspase-9和caspase-6蛋白相对表达水平将浓度为1×106mL-1各组细胞分别接种于直径为90 mm培养皿中,待细胞达到80%融合后,给予4.0 Gy X射线照射,再培养12 h,收集各处理组细胞,提取总蛋白。Western blotting主要步骤包括灌胶、电泳、转膜、剪膜、封闭、抗体孵育、曝光、显影和定影。采用Bio-Rad凝胶成像系统采集图像,其结果采用QuantityOne4.6.2软件进行分析。蛋白相对表达水平=(处理组基因灰度值/处理组内参灰度值)/(对照组基因灰度值/对照组内参灰度值)。
1.6 统计学分析采用SPSS19.0统计软件进行统计学分析。各组细胞中DR4、caspase-9和caspase-6 mRNA及蛋白相对表达水平以x± s 表示,组间样本均数比较采用单因素方差分析。以α=0.01为检验水准。
2 结 果 2.1 各组细胞中DR4、caspase-9和caspase-6 mRNA的表达水平与对照组比较,经4.0 Gy X射线照射后4 h,空质粒组、pEgr1-TRAIL组、 4.0 Gy组X射线、空质粒+4.0 GyX射线组和pEgr1-TRAIL+4.0 Gy X射线组细胞中DR4、caspase-9和caspase-6 mRNA表达水平均升高(P <0.01);8 h达最高值;其中不同时间pEgr1-TRAIL+4.0 Gy X射线组细胞中DR4、caspase-9和caspase-6 mRNA表达水平明显高于其他各组(P<0.01)。见表 1~3。
| (n=6,x± s ) | ||||
| Group | Expression level of DR4 mRNA | |||
| (t/h)4 | 8 | 12 | 24 | |
| Control | 1 | 1 | 1 | 1 |
| Empty plasmid | 5.46±0.34* | 9.52±0.29* | 7.54± 0.37* | 5.28±0.23* |
| pEgr1-TRAIL | 6.48±0.31* | 14.24±0.29* | 11.87±0.68* | 3.88±0.59* |
| 4.0 Gy X-ray | 4.96±0.54* | 7.49±0.37* | 5.87±0.47* | 2.94±0.38* |
| Empty plasmid+4.0 Gy X-ray | 13.51± 0.63* | 26.17±2.41* | 18.76±0.67* | 4.78±0.33* |
| pEgr1-TRAIL+4.0 GyX-ray | 22.31±0.59*△#○▲ | 109.49±3.67*△#○▲ | 45.16±0.69*△#○▲ | 8.78±0.28*△#○▲ |
| *P<0.01 vs control group;△P< 0.01 vs empty plasmid group;#P<0.01 vs pEgr1-TRAIL group;○P<0.01 vs4.0 Gy X-ray group;▲P<0.01 vsempty plasmid+4.0 Gy X-ray group. | ||||
| (n=6,x± s ) | ||||
| Group | Expression level of caspase-9 mRNA | |||
| (t/h)4 | 8 | 12 | 24 | |
| Control | 1 | 1 | 1 | 1 |
| Empty plasmid | 5.57±0.35* | 9.26±0.37* | 8.56±0.76* | 2.45±0.29* |
| pEgr1-TRAIL | 8.63±0.48* | 14.56±0.43* | 11.52±0.49* | 5.78±0.24* |
| 4.0 GyX-ray | 4.72±0.61* | 8.53±0.42* | 6.17±0.29* | 2.35±0.18* |
| Empty plasmid+4.0 Gy X-ray | 8.79±0.26* | 24.57±0.61* | 12.45±0.79* | 6.73±0.28* |
| pEgr1-TRAIL+4.0 Gy X-ray | 16.44±0.39*△#○▲ | 158.47±4.36*△#○▲ | 46.22±0.45*△#○▲ | 7.93 ±0.57*△#○▲ |
| *P<0.01 vs control group;△P<0.01 vs empty plasmid group;#P<0.01 vs pEgr1-TRAIL group; ○P<0.01 vs 4.0 Gy X-ray group;▲P<0.01 vs empty plasmid+4.0 Gy X-ray group | ||||
| (n=6,x± s ) | ||||
| Group | Expression level of caspase-6 mRNA | |||
| (t/h)4 | 8 | 12 | 24 | |
| Control | 1 | 1 | 1 | 1 |
| Empty plasmid | 6.59±0.44* | 12.78±0.24* | 13.59±0.45* | 7.48±0.57* |
| pEgr1-TRAIL | 10.44± 0.34* | 20.18±0.34* | 15.78±0.57* | 12.76±0.65* |
| 4.0 GyX-ray | 5.38±0.16* | 7.52±0.11* | 6.68±0.21* | 5.49±0.23* |
| Empty plasmid+4.0 Gy X-ray | 12.59±0.78* | 23.51±0.79* | 19.68±0.96* | 16.47±0.81* |
| pEgr1-TRAIL+4.0 Gy X-ray | 27.69±0.82*△#○▲ | 167.43±11.78*△#○▲ | 59.53±3.74*△#○▲ | 39.47±6.58*△#○▲ |
| *P<0.01 vs control group;△P<0.01 vs empty plasmid group;#P< 0.01 vs pEgr1-TRAIL group; ○P<0.01 vs 4.0 Gy X-ray group;▲P<0.01 vs empty plasmid+4.0 Gy X-ray group | ||||
4.0 Gy X射线照射后6 h,与对照组比较,其他各组MCF-7细胞中DR4、caspase-9和caspase-6蛋白相对表达水平开始升高,12 h后达峰值,随着时间的延长蛋白相对表达水平开始下降,但48 h仍高于正常值;与对照组比较,其他各组MCF-7细胞中蛋白相对表达水平由高到低的顺序为pEgr1-TRAIL+4.0 GyX射线组>空质粒+4.0 Gy X射线组>4.0 GyX射线组>pEgr1-TRAIL质粒组>空质粒组>对照组,其中pEgr1-TRAIL+4.0 GyX射线组细胞中DR4、caspase-9和caspase-6蛋白相对表达水平上升最明显。见图 1。
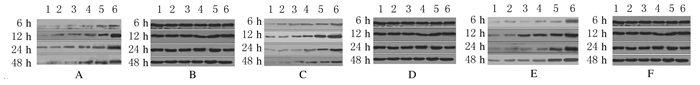
|
| Lane 1:Control group; Lane 2:Empty plasmid group; Lane 3:pEgr1-TRAIL group; Lane 4:4.0 Gy X-raygroup;Lane 5:Empty plasmid+4.0 Gy X-raygroup; Lane 6:pEgr1-TRAIL+4.0 Gy X-raygroup.A:DR4;C:Caspase-9;E:Caspase-6;B,D,F:GAPDH. 图 1 照射不同时间人乳腺癌MCF-7 细胞中DR4、caspase-9和caspase-6蛋白表达电泳图 Fig. 1 Electrophoregram of expressions of DR4,caspase-9, and caspase-6 proteins in MCF-7 cells in various groups after irradiated for different time |
本实验采用pEgr1-TRAIL重组质粒转染MCF-7细胞并联合电离辐射,观察MCF-7细胞死亡受体通路相关DR4、caspase-9和caspase-6mRNA和蛋白表达水平。本研究结果表明:4.0 Gy X射线照射后4 h,各组MCF-7细胞中DR4、caspase-9和caspase-6 mRNA和蛋白表达水平均开始升高,分别于8和12 h达高峰,其中pEgr1-TRAIL+4.0 Gy组细胞中mRNA和蛋白表达水平升高最明显,照射后48 h其mRNA和蛋白表达仍高于正常水平,提示pEgr1-TRAIL重组质粒联合电离辐射具有杀伤和诱导MCF-7细胞凋亡的作用。
细胞凋亡的通路主要包括两方面:一是启动死亡受体通路,二是启动线粒体凋亡通路[11]。本实验通过转染pEgr1-TRAIL重组质粒联合电离辐射启动了MCF-7细胞死亡受体通路,TRAIL可与细胞膜上的DR4结合,形成三聚体,激活caspase-9和caspase-6,使DR4、caspase-9和caspase-6基因和蛋白表达增强,促进了MCF-7细胞凋亡。
虽然TRAIL应用前景良好,并且具有杀伤肿瘤细胞而不杀伤正常细胞的特异性,但其治疗谱并不宽,一些肿瘤对TRAIL也具有抗拒性。电离辐射可杀伤肿瘤细胞,与TRAIL基因的联合应用已取得了明显的效果。研究[12]表明:电离辐射可使某些恶性肿瘤细胞对TRAIL表现出较高的敏感性,这是由于辐射诱导的死亡受体DR表达量升高,从而提高肿瘤细胞对TRAIL的敏感性。体内实验[13]表明:TRAIL和辐射的联合应用可使已建立起来的异种移植乳腺癌发生快速消退。
TRAIL基因可选择性地诱导肿瘤细胞凋亡,而对正常细胞不产生毒性,联合放疗可发挥杀灭肿瘤细胞的协同作用[14, 15]。目前,以TRAIL为基础的抗肿瘤药物研发取得成效,主要用于治疗乳腺癌、甲状腺癌、胰腺癌和结肠癌等实体瘤,其安全性和有效性得到肯定[16, 17]。本研究初步阐明了pEgr1-TRAIL重组质粒联合放疗对MCF-7细胞杀伤及诱导凋亡的作用,为乳腺癌治疗提供了新思路。
| [1] | Tait SW,Ichim G,Green DR. Die another way-non-apoptotic mechanisms of cell death [J].J Cell Sci,2014,127(Pt 10):2135-2144. |
| [2] | Chen YJ,Chi CW,Su WC,et al.Lapatinib induces autophagic cell death and inhibits growth of human hepatocellular carcinoma [J].Oncotarget,2014,5(13):4845-4854. |
| [3] | Bai L,Wang S.Targeting apoptosis pathways for new cancer therapeutics [J].Annu Rev Med,2014,65:139-155. |
| [4] | Lin Y,Wang X,Jin H.EGFR-TKI resistance in NSCLC patients:mechanisms and strategies [J].Am J Cancer Res,2014,4(5):411-435. |
| [5] | Bae JH,Shim JH,Cho YS.Chemical regulation of signaling pathways to programmed necrosis [J].Arch Pharm Res,2014,37(6):689-697. |
| [6] | Khaider NG,Lane D,Matte I,et al.Targeted ovarian cancer treatment:the TRAILs of resistance [J].Am J Cancer Res,2012,2(1):75-92. |
| [7] | Chunhacha P,Chanvorachote P.Roles of caveolin-1 on anoikis resistance in non small cell lung cancer [J].Int J Physiol Pathophysiol Pharmacol,2012,4(3):149-155. |
| [8] | Vanden Berghe T,Linkermann A,Jouan-Lanhouet S,et al.Regulated necrosis:the expanding network of non-apoptotic cell death pathways [J].Nat Rev Mol Cell Biol,2014,15(2):135-147. |
| [9] | Jonckheere N,Vincent A,van Seuningen I.Of autophagy and in vivo pancreatic carcinogenesis:the p53 status matters [J]. Clin Res Hepatol Gastroenterol,2014,38(4):423-425. |
| [10] | Bitto A,Lerner CA,Nacarelli T,et al.P62/SQSTM1 at the interface of aging,autophagy,and disease [J].Age (Dordr),2014,36(3):9626. |
| [11] | Sanli T,Steinberg GR,Singh G,et al.AMP-activated protein kinase (AMPK) beyond metabolism:a novel genomic stress sensor participating in the DNA damage response pathway [J].Cancer Biol Ther,2014,15(2):156-169. |
| [12] | 关 锋,龚平生,王志成,等.肿瘤坏死因子相关凋亡配体在肿瘤放射治疗中的作用[J].中华放射医学与防护杂志,2014,34(3):231-234. |
| [13] | Shiloh R,Bialik S,Kimchi A.The DAPK family:a structure-function analysis [J].Apoptosis,2014,19(2):286-297. |
| [14] | Suman S,Das TP,Reddy R,et al.The pro-apoptotic role of autophagy in breast cancer [J].Br J Cancer,2014,111(2):309-317. |
| [15] | Ettinger DS,Akerley W,Borghaei H,et al.National comprehensive cancer network:Non-small cell lung cancer,version 2.2013 [J].J Natl Compr Canc Netw,2013,11(3):645-653. |
| [16] | Hsu CL,Chen JH,Chen KY,et al.Advanced non-small cell lung cancer in the elderly:The impact of age and comorbidities on treatment modalities and patient prognosis [J].J Geriatr Oncol,2015,6(4):38-45. |
| [17] | 张卫芳,孙 燕,张伟杰,等.TM4SF1在人乳腺癌MCF-7和MDA-MB-231中的表达及其对细胞增殖、迁移能力的影响[J].郑州大学学报:医学版,2016,51(2):240-244. |
 2016, Vol. 42
2016, Vol. 42


