扩展功能
文章信息
- 邵婉婷, 付彤, 武盼盼, 吴迪, 李嗣杰, 郑超, 范志民
- SHAO Wanting, FU Tong, WU Panpan, WU Di, LI Sijie, ZHENG Chao, FAN Zhimin
- Her-2阳性乳腺癌患者曲妥珠单抗联合新辅助化疗的效果评价及其预后影响因素分析
- Evaluation on efficacy of trastuzumab combined with neoadjuvant chemotherapy in Her-2 positive breast cancer patients and analysis of influencing factors of prognosis
- 吉林大学学报(医学版), 2016, 42(02): 351-357
- Journal of Jilin University (Medicine Edition), 2016, 42(02): 351-357
- 10.13481/j.1671-587x.20160230
-
文章历史
- 收稿日期: 2015-11-01
人类表皮生长因子受体2(human epidermal growth factor receptor-2,Her-2)在约15%~20%的浸润性乳腺癌患者中存在基因扩增或Her-2蛋白过表达[1]。在Her-2靶向治疗可用之前,Her-2阳性乳腺癌患者较Her-2阴性乳腺癌患者面临着更差的预后,此类患者的肿瘤生物学行为特征为恶性程度高、无病生存期(disease- free survival,DFS)和总生存期(overall survival,OS)短、具有更高的远处转移率和更高的死亡率[1, 2]。曲妥珠单抗是一种靶向作用于Her-2蛋白细胞外区域的药物,是一种重组DNA衍生的人源化单克隆抗体[3]。目前,新辅助化疗是局部晚期乳腺癌的标准方法[4]。国际大型临床试验[5]结果表明:将新辅助化疗联合曲妥珠单抗用于Her-2阳性乳腺癌可改善预后,提高患者的生存率。但该药价格昂贵,国内临床应用的并不广泛,相关临床报道不多。本研究回顾性分析曲妥珠单抗联合新辅助化疗用于Her-2阳性乳腺癌患者中的疗效和预后评价。
1 资料与方法 1.1 临床资料本研究回顾性分析了2005年9月—2013年12月于吉林大学白求恩第一医院乳腺外科接受新辅助化疗的112例Her-2阳性乳腺癌患者的临床资料。通过乳腺癌数据库对患者的基本信息、临床特点、治疗方案、术后结果和随访情况进行记录。所有患者均经过临床查体、辅助检查及组织学活检确诊为浸润性乳腺癌。治疗前均接受相关检验检查评估是否发生远处转移以及各脏器功能是否耐受化疗。
1.2 病理学评估所有患者均接受2次病理学检测,即新辅助化疗前活检组织及手术后切除组织病理检测。所有患者的病理类型均为浸润性导管癌。应用免疫组织化学染色技术分析雌激素受体(estrogen receptor,ER)、孕激素受体(progesterone receptor,PR)和Her-2表达。在所有肿瘤细胞中超过1%的强度均视为ER或PR阳性。Her-2染色强度由0~3+来评估,2+及3+需要行荧光原位杂交技术(FISH)检测,进一步确定基因是否扩增。Her-2阳性的评定标准为Her-2 2+且FISH检测为阳性、Her-2 3+以及Her-2 3+且FISH检测为阳性。根据2次病理检测情况,应用Miller and Payne(MP)分级评估化疗疗效,分为1~5级。MP分级为5级(原发病灶无浸润性癌细胞残留或有原位癌残留)且术后腋窝淋巴结阴性的患者视为病理完全缓解(pathological complete remission,PCR)。
1.3 治疗方案根据患者保乳意愿及肿物临床分期考虑是否接受新辅助化疗,所有患者的化疗方案均依据NCCN指南,应用曲妥珠单抗的患者行TCH方案(多西他赛、卡铂和曲妥珠单抗),对于拒绝使用曲妥珠单抗的患者采用蒽环类药物联合紫衫类药物,即TAC方案(多西他赛/紫杉醇、吡柔比星和环磷酰胺),所有方案治疗后均预防性应用粒细胞集落刺激因子。化疗周期为21d,共计6个疗程。根据患者个人意愿、肿瘤对化疗反应以及患者耐受等情况,新辅助化疗疗程有所不同。所有患者均接受手术治疗,手术方式的选择依据患者的意愿、肿物的特点及临床经验丰富的外科医生的评估。保乳手术患者连续切缘阳性即行乳房全切手术,术中及术后前哨淋巴结阳性则行腋窝淋巴结清扫术。依据NCCN指南对于肿物直径>5cm、腋窝淋巴结阳性、切缘阳性、保乳手术等情况建议患者行放疗。不论术前或术后病理结果,ER或PR阳性的患者均应接受5年内分泌治疗。术后根据患者病情需要及意愿选择是否接受放疗、内分泌治疗及分子靶向治疗。
曲妥珠单抗首次以8 mg·kg-1剂量给药,其后每次剂量为6 mg·kg-1,每3周返院化疗前均需复查心脏彩超评估心功能,接受治疗期间,未见发生左心室射血分数降低等心功能异常的患者。
1.4 患者分类本研究除外曲妥珠单抗治疗中出现输液反应、心功能异常及不能耐受药物的患者,接受靶向治疗均满1年;除外双侧乳腺癌患者及初诊即发现远处转移的患者。将112例新辅助化疗患者根据是否应用曲妥珠单抗治疗分为靶向联合化疗组23例(20.54%)和单纯化疗组89例(79.46%)。
1.5 随访情况术后每1年进行一次电话随访,中位随访时间为28个月(12~70个月),靶向联合化疗组中位随访时间为30个月(16~66个月),单纯化疗组中位随访时间为28个月(12~70个月)。局部复发是指临床查体、辅助检查、活组织病理学检查证实的治疗侧乳房、区域淋巴结(腋窝、内乳和锁骨上下)复发,远处转移指辅助检查或活组织病理学检查证实有远处转移病灶。DFS是初次治疗到第1次复发(局部复发、远处转移或对侧乳房发病)的时间间隔,OS是指从确诊之日到不论任何原因的死亡日期或最后随访日期。
1.6 统计学分析采用SPSS19.0统计软件进行统计分析。计数资料组间比较采用χ2检验,生存分析采用Kaplan-Meier法,生存曲线应用Log-rank检验,多因素预后分析采用Cox回归分析。以P<0.05为差异具有统计学意义。
2 结果 2.1 112例Her-2阳性乳腺癌患者的生存分析112例患者的临床特征见表 1。患者中位年龄为50.5岁(29~65岁),均为女性。靶向联合化疗组患者中位年龄为49岁(33~65岁),单纯化疗组患者中位年龄为51岁(29~63岁)。总体患者的中位OS为62.13个月,中位DFS为56.58个月。靶向联合化疗组所有患者均未出现复发及转移,单纯化疗组有5例(5.6%)患者出现复发,占所有患者的4.5%,2组比较差异无统计学意义(P=0.245)。单纯化疗组有19例(21.3%)患者出现转移,占所有患者的17.0%,2组比较差异有统计学意义(P=0.015),肝转移、肺转移、骨转移以及超过2处转移患者均占21.05%,脑转移为10.53%,其他为5.26%。单纯化疗组中有14例(15.7%)患者死于肿瘤相关的任何原因。靶向联合化疗组PCR4例,PCR率为17.4%;单纯化疗组PCR12例,PCR率为13.5%,2组比较差异无统计学意义(P=0.695)。本文作者以激素受体(hormone receptor,HR)状态为分层因素对HR情况与PCR的关系进行了分析:HR阳性患者中,获得PCR的患者为5例(10.0%);HR阴性患者中,获得PCR为11例(29.0%),虽然HR阴性患者更易获得PCR,但2组间比较差异无统计学意义(P=0.191)。HR阴性患者术后病理回报MP分级为5级的18例(32.7%),HR阳性患者则为5例(10.9%),2组比较差异有统计学意义(P=0.009)。
| [n(η/%)] | |||||
| Clinical characteristic | Without trastuzumab ( n=89) | With trastuzumab ( n=23) | Total ( n=112) | χ 2 | P |
| Age(year) | |||||
| <50 ≥50 | 38(42.7)51(57.3) | 13(56.5) 10(43.5) | 51(45.5) 61(54.5) | 1.409 | 0.235 |
| Menopause | |||||
| Premenopausal Postmenopausal | 41(46.1)48(53.9) | 13(56.5)10(43.5) | 54(48.2) 58(51.8) | 0.800 | 0.371 |
| Family history | |||||
| Yes No | 2(2.2) 87(97.8) | 5(21.7) 18(78.3) | 7(6.3) 105(93.8) | 11.851 | 0.001 |
| HR | |||||
| Negative Positive | 47(52.8)42(47.2) | 15(65.2) 8(34.8) | 62(55.4) 50(44.6) | 1.139 | 0.286 |
| Tumor size | |||||
| cT1 cT2 cT3 cT4 | 7(7.9)58(65.2)15(16.9)9(10.1) | 6(26.1) 13(56.5) 3(13.0) 1(4.3) | 13(11.6) 71(63.4) 18(16.1)10(8.9) | 6.289 | 0.098 |
| Lymph node status | |||||
| cN0 cN1 cN2 cN3 | 23(25.8) 31(34.8)29(32.6) 6(6.7) | 6(26.1) 8(34.8) 7(30.4) 2(8.7) | 29(25.9) 39(34.8) 36(32.1) 8(7.1) | 0.124 | 0.989 |
| Clinical stage | |||||
| Stage 1 | 6(6.7) | 1(4.3) | 7(6.3) | ||
| Stage 2 | 40(44.9) | 11(47.8) | 51(45.5) | ||
| Stage 3 | 43(48.3) | 11(47.8) | 54(48.2) | 0.202 | 0.904 |
| Breast operation | |||||
| Breast-conserving Mastectomy | 10(11.2)79(88.8) | 6(26.1)17(73.9) | 16(14.3) 96(85.7) | 3.292 | 0.070 |
| Axillary operation | |||||
| SLNB ALND | 4(4.5) 85(95.5) | 1(4.3)22(95.7) | 5(4.5)107(95.5) | 0.001 | 0.976 |
| Chemotherapy courses before operation | |||||
| 1-3 ≥4 | 13(14.6)76(85.4) | 3(13.0)20(87.0) | 16(14.3) 96(85.7) | 0.036 | 0.849 |
| Radiotherapy | |||||
| Yes No | 64(71.9)25(28.1) | 20(87.0) 3(13.0) | 84(75.0) 28(25.0) | 2.207 | 0.137 |
| Endocrinotherapy | |||||
| Yes No | 36(40.4)53(59.6) | 8(34.8)15(65.2) | 44(39.3) 69(60.7) | 0.246 | 0.620 |
| MP classification | |||||
| 1-2 3-4 5 N.A. | 19(24.4)42(53.8)17(21.8)11 | 2(8.7)15(65.2)6(26.1) | 21(20.8) 57(56.4) 23(22.8) | 2.647 | 0.266 |
| Node status after operation | |||||
| pN0 pNx | 38(42.7) 51(57.3) | 13(56.5) 10(43.5) | 51(45.5) 61(54.5) | 1.409 | 0.235 |
| PCR | |||||
| Yes | 12(14.1) | 4(17.4) | 16(14.8) | ||
| No | 73(85.9) | 19(82.6) | 92(85.2) | 0.154 | 0.695 |
| N.A. | 4 | ||||
| cT: Clinical tumor stage; cN: Clinical node stage; N.A.: Not available; SLNB: Sentinel lymph node biopsy; ALND: Axillary lymph node dissection. | |||||
所有患者的3年DFS和OS分别为85.2%、92.4%,5年DFS和OS分别为79.3%、86.3%。单纯化疗组患者的3年DFS和OS分别为81.6%、90.5%,5年DFS和OS分别为74.3%、83.0%。单纯化疗组患者的DFS明显低于靶向联合化疗组(P=0.012),见图 1。单纯化疗组患者的OS虽然低于靶向联合化疗组,但差异无统计学意义(P=0.064),见图 2。在分层分析中,年龄<50岁(P=0.047)、绝经前(P=0.031)、临床分期Ⅲ期(P=0.013)、HR阴性(P=0.017)、术后腋窝淋巴结阳性(P=0.017)、接受放疗(P=0.026)、未接受内分泌治疗(P=0.015)、未获得PCR(P=0.018)、行腋窝淋巴结清扫术(P=0.012)及乳房全切手术(P=0.027)等情况下,靶向联合化疗组与单纯化疗组DFS比较差异有统计学意义,即靶向联合化疗组患者预后情况较好;而OS只在临床分期Ⅲ期患者中比较差异有统计学意义(P=0.049)。
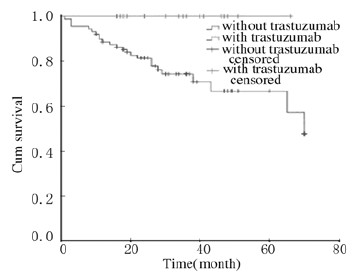
|
| 图1 靶向联合化疗组和单纯化疗组Her-2阳性乳腺癌患者DFS曲线 Fig.1 DFS curves of Her-2 positive breast cancer patients in combined treatment group and single chemotherapy group |

|
| 图2 靶向联合化疗组和单纯化疗组Her-2阳性乳腺癌患者OS曲线 Fig.2 OS curves of Her-2 positive breast cancer patients in combined treatment group and single chemotherapy group |
单因素及多因素赋值情况:年龄<50岁为1,年龄≥50岁为2;未绝经为0,已绝经为1;cT分期:cT1为1,cT2为2,cT3为3,cT4为4;cN分期:cN0为0,cN1为1,cN2为2,cN3为3;放疗:Yes为1,No为0;MP分级:1~2级为1,3~4级为2,5级为3,N.A.为-1;术后淋巴结情况:pN0为0,pN1为1。采用Cox回归进行单因素分析时发现:Her-2阳性乳腺癌患者原发肿物大小、初诊时区域淋巴结情况及术后淋巴结情况是DFS的影响因素,而原发肿物大小、初诊时区域淋巴结情况、术后淋巴结情况和MP分级情况是OS的影响因素。见表 2。
| Item | DFS | OS | ||
| HR(95%CI) | P | HR(95%CI) | P | |
| Age | 0.92(0.41-2.06) | 0.835 | 0.54(0.18-1.66) | 0.283 |
| Menopause | 0.74(0.32-1.68) | 0.465 | 0.62(0.20-1.90) | 0.400 |
| Tumor size | 2.91(1.80-4.71) | 0.000 | 3.73(1.96-7.12) | 0.000 |
| Node status | 2.69(1.64-4.43) | 0.000 | 4.69(2.16-10.18) | 0.000 |
| Radiotherapy | 0.63(0.26-1.55) | 0.318 | 0.49(0.16-1.51) | 0.214 |
| MP classification | 0.85(0.61-1.17) | 0.315 | 0.65(0.44-0.97) | 0.035 |
| Node status after operation | 4.50(1.53-13.17) | 0.006 | 10.48(1.36-80.69) | 0.024 |
采用Cox回归进行多因素分析时发现:原发肿物大小、初诊时区域淋巴结情况、是否接受放疗是Her-2阳性乳腺癌患者DFS及OS的独立影响因素。T、N分期越高患者复发及预后风险越高;未接受放疗患者复发及预后风险也较高。见表 3。
| Item | DFS | OS | ||
| HR(95%CI) | P | HR(95%CI) | P | |
| Age | 1.85(0.43-8.02) | 0.413 | 0.27(0.13-5.46) | 0.394 |
| Menopause | 0.28(0.06-1.30) | 0.104 | 1.30(0.07-25.77) | 0.861 |
| Tumor size | 3.70(2.10-6.51) | 0.000 | 4.36(1.84-10.33) | 0.001 |
| Node status | 2.98(1.59-5.57) | 0.001 | 4.45(1.77-11.18) | 0.002 |
| Radiotherapy | 0.31(0.11-0.85) | 0.023 | 0.15(0.04-0.60) | 0.007 |
| MP classification | 1.53(0.96-2.42) | 0.072 | 1.25(0.63-2.49) | 0.529 |
| Node status after operation | 2.43(0.72-8.15) | 0.151 | 4.39(0.48-39.81) | 0.189 |
目前,新辅助化疗在乳腺癌的综合治疗中占有重要地位。相对于辅助化疗,新辅助化疗能够降低临床分期,增加患者获得保乳或者手术切除的机会,同时可以有效地观察药物敏感性[4]。Her-2过表达患者具有生物学行为恶性度高、生存期短、预后差等特点[5]。随着曲妥珠单抗在临床应用的日益广泛,Her-2阳性乳腺癌患者的DFS和OS得到明显改善[6]。自2005年以来,将曲妥珠单抗联合新辅助化疗用于Her-2阳性乳腺癌患者的治疗也取得了重大进展[7, 8]。Buzdar等[7]首次报道了42例接受紫杉类和蒽环类治疗、并评估随机接受或不接受曲妥珠单抗治疗患者的疗效,以PCR率进行评价,接受或不接受曲妥珠单抗治疗患者的PCR率分别为65.2%和26.0%(P=0.016)。随后该试验在2007年更新的数据证明接受曲妥珠单抗治疗患者的DFS显著提高[9]。
在NOAH试验[10]中,235例Her-2阳性局部晚期乳腺癌和炎性乳腺癌接受新辅助治疗,并随机接受或不接受曲妥珠单抗治疗,结果表明:与未接受曲妥珠单抗治疗组比较,接受曲妥珠单抗治疗组患者的PCR率(43%vs22%,P=0.000 7)、3年DFS率(71%vs56%,HR=0.59,P=0.013)均显著提高。2013年经过平均时间为5.4年的随访后,结果表明:接受曲妥珠单抗治疗组患者的OS率也有所提高(73.5%vs62.9%,HR=0.66,P=0.055)[11]。在本研究中,靶向联合化疗组的PCR为17.4%,高于单纯化疗组的13.5%,但由于入组病例较少,2组PCR率并没有明显差异。本研究中,靶向联合化疗组的DFS显著高于单纯化疗组;由于曲妥珠单抗价格较高,且近年来用药人数增加,所以靶向联合化疗组患者随访时间较短,使得单纯化疗组的OS明显高于靶向联合化疗组,但差异无统计学意义,与以上研究[9, 10]结果类似。
目前,关于HR状态在新辅助化疗联合曲妥珠单抗治疗中的疗效预测价值仍未明确,Peintinger等[12]研究表明:HR阳性的乳腺癌患者的PCR率较高,而另外一些研究[13, 14]结果则相反。NOAH试验[15]的亚组分析结果表明:PR阴性患者接受新辅助化疗联合曲妥珠单抗治疗后PCR率较高。而本研究中,HR阴性患者更易获得PCR,但2组比较差异无统计学意义。2003年Ogston等[16]报道了新辅助化疗后病理评价病灶缓解程度的方法—MP分级,该方法以病灶密度改变情况评价疗效,为临床判断病理缓解情况提供了标准。本研究结果显示:HR阴性患者术后病理回报MP分级为5级的18例(32.7%),HR阳性患者则为5例(10.9%),两者比较差异有统计学意义。因此,本文作者认为将MP分级与PCR情况结合可以用来预测新辅助化疗联合曲妥珠单抗治疗对乳腺癌患者预后的影响。
心功能障碍一直是曲妥珠单抗相关毒性的关注热点。有关安全性研究数据表明:约6.8%的单纯接受化疗组患者的左室射血分数较基线降低10%~15%,而在曲妥珠单抗组中的发生率为13.3%[17]。MD安德森癌症中心的相关研究[7]发现:有26%的单纯接受化疗患者左室射血分数降低10%,而曲妥珠单抗联合化疗组为30%,但在随后的随访中发现曲妥珠单抗联合化疗组患者心功能障碍的发生率并没有增加。NOAH试验[15]的结果显示:大多数情况下曲妥珠单抗对心脏的影响均可逆转。国内研究[18, 19, 20]也认为:曲妥珠单抗联合新辅助化疗组患者的耐受性良好。因此,本文作者认为在对患者严格管理和监控下,曲妥珠单抗联合新辅助化疗用于Her-2阳性乳腺癌患者是安全可行的。
综上所述,对于HR阴性Her-2阳性乳腺癌患者,曲妥珠单抗联合新辅助化疗后乳房病灶可获得显著缓解,即MP分级为5级。曲妥珠单抗联合新辅助化疗治疗Her-2阳性乳腺癌患者,可以降低患者局部复发,显著提高患者的DFS,对患者的预后具有积极的意义。T、N分期及是否接受放疗是Her-2阳性乳腺癌患者DFS及OS的独立预后因素。本研究虽比较全面地评估了曲妥珠单抗联合新辅助化疗的疗效及对患者预后的影响,但是入组例数不足,随访时间尚短,尚需进一步扩大病例数,延长随访时间,以获得更加详细的临床资料。
| [1] | Slamon DJ,Godolphin WA,Jones LA,et al.Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer[J].Science,1989,244(4905):707-712. |
| [2] | Perez EA,Romond EH,Suman VJ,et al.Trastuzumab Plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer:planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831[J].J Clin Oncol,2014,32(33):3744-3752. |
| [3] | Slamon DJ,Leyland-Jones B,Shak S,et al.Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2[J].N Engl J Med,2001,344(11):783-792. |
| [4] | van der Hage JA,van de Velde CJH,Julien JP,et al.Preoperative chemotherapy in primary operable breast cancer:Results from the European Organization for Research and Treatment of Cancer trial 10902[J].J Clin Oncol,2001,19(22):4224-4237. |
| [5] | Valachis A,Mauri D,Polyzos NP,et al.Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer:A systematic review and meta-analysis[J].Breast,2011,20(6):485-490. |
| [6] | Demonty G,Bernard-Marty C,Puglisi F,et al.Progress and new standards of care in the management of HER-2 positive breast cancer[J].Eur J Cancer,2007,43(3):497-509. |
| [7] | Buzdar AU,Ibrahim NK,Francis D,et al.Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab,paclitaxel,and epirubicin chemotherapy:Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer[J].J Clin Oncol,2005,23(16):3676-3685. |
| [8] | Chia SK.Neoadjuvant and adjuvant therapy for her2 positive disease[J].Am Soc Clin Oncol Educ Book,2015,35:e41-e48. |
| [9] | Buzdar AU,Valero V,Ibrahim NK,et al.Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil,epirubicin,and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer:An update of the initial randomized study population and data of additional patients treated with the same regimen[J].Clin Cancer Res,2007,13(1):228-233. |
| [10] | Gianni L,Eiermann W,Semiglazov V,et al.Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone,in patients with HER2-positive locally advanced breast cancer (the NOAH trial):a randomised controlled superiority trial with a parallel HER2-negative cohort[J].Lancet,2010,375(9712):377-384. |
| [11] | Gianni L,Eiermann W,Semiglazov V,et al.Follow-up results of NOAH,a randomized phase Ⅲ trial evaluating neoadjuvant chemotherapy with trastuzumab (CT plus H) followed by adjuvant H versus CT alone,in patients with HER2-positive locally advanced breast cancer[J].J Clin Oncol,2013,31(15):503. |
| [12] | Peintinger F,Buzdar AU,Kuerer HM,et al.Hormone receptor status and pathologic response of HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab[J].Ann Oncol,2008,19(12):2020-2025. |
| [13] | Limentani SA,Brufsky AM,Erban JK,et al.Phase Ⅱ study of neoadjuvant docetaxel,vinorelbine,and trastuzumab followed by surgery and adjuvant doxorubicin plus cyclophosphamide in women with human epidermal growth factor receptor 2-overexpressing locally advanced breast cancer[J].J Clin Oncol,2007,25(10):1232-1238. |
| [14] | Guarneri V,Broglio K,Kau SW,et al.Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors[J].J Clin Oncol,2006,24(7):1037-1044. |
| [15] | Gianni L,Eiermann W,Semiglazov V,et al.Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH):follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort[J].Lancet Oncol,2014,15(6):640-647. |
| [16] | Ogston KN,Miller ID,Payne S,et al.A new histological grading system to assess response of breast cancers to primary chemotherapy:prognostic significance and survival[J].Breast,2003,12(5):320-327. |
| [17] | Chang HR.Trastuzumab-based neoadjuvant therapy in patients with her2-positive breast cancer[J].Cancer,2010,116(12):2856-2867. |
| [18] | 廖宁,张国淳,李学瑞,等.新辅助紫杉醇联合曲妥珠单抗在表皮生长因子受体2阳性可手术乳腺癌患者中的疗效及安全性评价[J].中华肿瘤杂志,2010,32(7):544-547. |
| [19] | 姚恒,赵玲,李索妮,等.紫杉醇脂质体对乳胶癌细胞SK-BR-3的放疗增敏作用及其机制[J].西安交通大学学报:医学版,2015,36(5):628-633. |
| [20] | 曹志宇,何建苗,杨波,等.局部晚期乳腺癌新辅助化疗联合保乳手术的临床疗效观察:附81例报告[J].解放军医学杂志,2015,40(6):488-491. |
 2016, Vol. 42
2016, Vol. 42


