扩展功能
文章信息
- 赵利军, 李开济, 卢秋玲, 门秀丽
- ZHAO Lijun, LI Kaiji, LU Qiuling, MEN Xiuli
- 缺血后处理对大鼠肢体缺血再灌注后肺损伤的保护作用及其机制
- Protective effect of ischemia postconditioning on lung injury after limb ischemia reperfusion in rats and its mechanism
- 吉林大学学报(医学版), 2016, 42(02): 255-259
- Journal of Jilin University (Medicine Edition), 2016, 42(02): 255-259
- 10.13481/j.1671-587x.20160213
-
文章历史
- 收稿日期: 2015-06-30
国内外大量研究[1, 2]证实:肢体缺血再灌注(limb ischemia reperfusion,LIR)可引起机体其他远隔器官的损伤,其中肺脏是最容易受累的器官之一,全身播散性的炎症反应是这种远位器官损伤的重要机制之一。近年来,在再灌注损伤的防治领域,缺血后处理(ischemic postconditioning,I-postC)是备受关注的器官保护措施[3, 4],即在缺血组织得到长时间的血流再灌注之前,先进行一次或数次短暂的缺血再灌注,可减轻随后持续再灌注引起的组织损伤。研究[5]显示:此保护作用同样适用于远隔器官及远隔后处理,但关于此保护作用的具体机制国内外尚未见详细而深入的报道。本实验以炎症反应为切入点,观察大鼠肢体I-postC对LIR后肺损伤的影响,探讨I-postC的肺脏保护机制,为临床再灌注损伤的防治提供理论依据。
1 材料与方法 1.1 实验动物和分组健康清洁级雄性Wistar大鼠24只,体质量200~250 g,购自河南医科大学动物中心,动物合格证号:SYXK(冀)2010-0038。大鼠随机分为3组(每组8只):对照组、缺血再灌注组(IR组)和I-postC组。动物术前24 h禁食, 自由饮水。
1.2 模型制备和标本采集以本室常规应用的止血带法复制大鼠LIR动物模型[1],即使用标准化弹性的橡皮带环绕结扎大鼠双后肢根部阻断血流4h,然后松解橡皮带恢复血流灌注4h。对照组大鼠松弛结扎双后肢不阻断血流,I-postC组大鼠在血流再灌注前,先行缺血5 min-再灌注5 min,重复3次,再恢复血流灌注4h。各组大鼠在再灌注4h时经腹主动脉取血约0.5 mL(隔绝空气,即将穿刺针头插入橡皮塞内)立即送血气分析,再取肝素抗凝血4 mL,经3500 r·min-1离心15 min,所得血浆标本分装在干净的Eppendorf 管中,-70℃保存备用。同时立即开胸取出肺脏,迅速液氮冷冻,-70℃储藏备用。此外,每组随机取3份右肺中叶,切取2/3迅速投入10%甲醛溶液中固定,待石蜡包埋后制备光镜切片。另外1/3的组织块立即投入冷的4%戊二醛-磷酸缓冲液,后经处理后制备电镜切片。
1.3 指标检测1血液CD18阳性细胞百分率:取肝素抗凝血4 mL,Percoll液分离大鼠血液中的中性粒细胞(neutrophils,PMN),流式细胞仪测定PMN中CD18-FITC标记的阳性细胞百分率。2血浆可溶性细胞间黏附分子1(soluble intercellular adhesion molecule-1,sICAM-1)、P-选择素(P-selectin)及肺组织髓过氧化物酶(myeloperoxidase,MPO)活性、细胞间黏附分子-1(intercellular adhesion molecule-1,ICAM-1)和p-selectin水平:以冻存的组织匀浆和血清标本为样品,按照试剂盒(厦门惠嘉生物科技有限公司)操作说明检测。3动脉血氧分压(PaO2)和动脉血二氧化碳分压(PaCO2):穿刺腹主动脉取血约0.5mL,立即隔绝空气,经雅培血气分析仪( i-STAT 300)检测PaO2和PaCO2水平。4肺组织光镜观察:切取的肺组织经10%中性甲醛固定,常规脱水,包埋,切片,脱蜡,HE染色,透明,封片。5肺组织电镜观察:切取的肺组织立即投入冷的4%戊二醛-磷酸缓冲液,切成1 mm×1 mm×1 mm的小块预固定,后转入2.5%戊二醛中固定,经丙酮脱水、环氧树脂618包埋、超薄切片、染色后在透射电镜下观察肺组织的超微结构。
1.4 统计学分析采用SPSS 17.0 统计软件进行统计学分析。各组大鼠血浆sICAM-1和P-selectin水平、PaO2和PaCO2检测结果、肺组织中MPO活性、ICAM-1和P-selectin水平等以x[TX-]±s表示,组间均数比较采用单因素方差分析。以P<0.05为差异有统计学意义。
2 结 果 2.1 各组大鼠血液中CD18阳性细胞数与对照组比较,IR组大鼠外周血中CD18阳性细胞百分率(14.83%±3.72%)明显升高(P<0.01),而I-postC组大鼠外周血中CD18阳性细胞百分率(10.02%±2.12%)则低于IR组(P<0.01)。
2.2 各组大鼠血浆sICAM-1和P-selectin水平与对照组比较,IR组大鼠血浆sICAM-1及P-selectin水平明显升高了132.23%及160.00%(P<0.01);与IR组比较,I-postC组大鼠血浆sICAM-1及P-selectin水平分别降低了26.69%及32.05%(P<0.01)。见表 1。
| [x±s,ρB/(μg·L-1)] | ||
| Group | sICAM-1 | P-selectin |
| * P<0.01 compared with control group;△P<0.01 compared with IR group. | ||
| Control | 1.21±0.10 | 0.30±0.04 |
| IR | 2.81±0.42* | 0.78±0.08* |
| I-postC | 2.06±0.30*△ | 0.53±0.06*△ |
与对照组比较,IR组大鼠肺组织中MPO活性、ICAM-1和P-selectin水平分别升高156.92%、138.53%和245.37%(P<0.01);与IR组比较,I-postC组大鼠肺组织中MPO活性、ICAM-1和P-selectin水平分别降低17.37%、37.26%和10.24%(P<0.01)。见表 2。
| (n=8,x±s) | |||
| Group | MPO | ICAM-1 | P-selectin |
| *P<0.01 compared with control group;△P<0.01 compared with IR group. | |||
| Control | 0.65±0.11 | 12.25±2.25 | 3.72±1.12 |
| IR | 1.67±0.30* | 29.22±4.78* | 12.85±1.98* |
| I-postC | 1.38±0.13*△ | 18.33±1.40*△ | 7.53±1.24*△ |
与对照组比较,IR组大鼠PaO2和PaCO2均明显降低,分别降低了32.36%和24.74%(P<0.01),损伤加重;与IR组比较,I-postC组大鼠PaO2和PaCO2均明显升高,分别升高了19.53%和17.33%(P<0.01)。见表 3。
| (n=8,x±s,P/kPa) | ||
| Group | PaO2 | PaCO2 |
| .*P<0.01 compared with control group;△P<0.01 compared with IR group. | ||
| Control | 95.25±1.89 | 42.64±1.79 |
| IR | 64.43±3.76* | 32.09±1.60* |
| I-postC | 77.01±4.92*△ | 37.65±1.61*△ |
与对照组比较,IR组大鼠肺组织水肿,肺泡隔增宽,毛细血管扩张充血,血管周围间隙增大,血管床中有大量炎性细胞聚集、附壁,其中除有PMN外,还有淋巴细胞及单核细胞,部分肺泡腔中可见到出血和蛋白渗出物,并伴有局限性肺不张。I-postC组大鼠上述病理学改变有所减轻。见图 1(插页四)。

|
| A: Control group? B: I/R group; C: I-postC group. 图1 光镜下各组大鼠肺组织形态学(HE, ×200) Fig.1 Morphology of lung tissue of rats in various groups under light microscope (HE,×200) |
电镜下,对照组大鼠肺组织凋亡细胞数稀少,组织细胞结构完整;与对照组比较,IR组大鼠肺泡毛细血管内皮细胞、Ⅰ型肺泡上皮细胞水肿变性,肺泡壁增厚,Ⅱ型肺泡上皮细胞膜微绒毛减少,线粒体肿胀、嵴减少,且胞质中板层小体明显减少,肺间质水肿,毛细血管腔内PMN堵塞。破坏细胞数明显增多,可见于肺泡上皮细胞、支气管上皮细胞、肺血管内皮细胞,组织损伤较重。与IR组比较,I-postC组大鼠肺组织破坏细胞数减少,组织损伤减轻。见图 2。
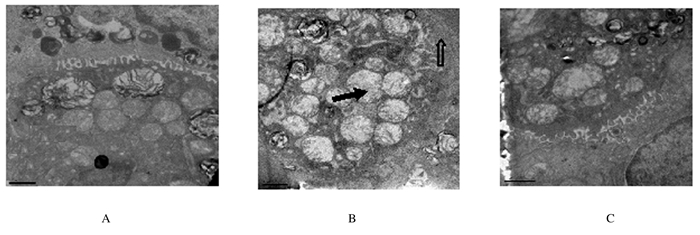
|
| A:Control group;B: I/R group ;C: I-postC group. The hollow arrow showed the decreasing of alveolar epithelial cell membrane microvilli thickening of alveolar walls; The solid arrow showed alveolar epithelial cell mitochondria swelling and cristae decreasing and cytoplasmic medium plate layer corpuscle decreasing. 图2 电镜下各组大鼠肺组织形态学 (Bar=1μm) Fig.2 Morphology of lung tissue of rats in various groups under electron microscope(Bar=1μm) |
LIR引起远隔器官损伤的机制十分复杂,其中过度的炎症反应是极为重要的机制之一[6]。肢体的缺血性损伤及血流再灌注中生成的多种体液因子可激活血液中的PMN,且随着再灌注过程的持续,此作用被不断加强[7, 8]。过量过度激活的PMN在肺组织内大量聚集、浸润和清除障碍是导致肺损伤的中心环节[9]。
MPO是PMN嗜天青颗粒中含量较高的酶,组织中MPO活性的高低可定量反应组织PMN的浸润程度[10]。活化的PMN可分泌弹性蛋白酶分解胶原,破坏肺结缔组织、肺泡上皮和血管内皮细胞基底膜等。在细胞间黏附分子(intercellular adhesion molecular,ICAM)的介导下,激活的PMN还可与血管内皮细胞相互黏附,这与PMN变形能力下降和肺微血管通透性增加引起的血浆外渗一起,成为加重肺微血管血流动力学紊乱的重要机制[11]。ICAM-1是细胞表面的一类跨膜糖蛋白,主要参与炎症和免疫反应,体内分布广泛,特别是在肺毛细血管内皮细胞、Ⅰ型与Ⅱ型肺泡上皮细胞及肺泡巨噬细胞上[12]。ICAM-1可与白细胞表面存在的黏附分子配体CD18分子结合,引起白细胞功能的活化和从血管游出,进入组织参与炎症反应[4]。P-selectin 是黏附分子选择素家族的重要成员,通常储存于内皮细胞的分泌颗粒( Weibe-l Palade 小体) 和血小板的A颗粒中[13]。当血管内皮细胞和血小板活化后,迅速表达于细胞表面,在介导白细胞与血管壁接触中发挥着重要的作用[14]。
本实验中LIR后,血浆和肺组织的ICAM-1及P-selectin 水平明显升高,提示全身过度炎症反应及肺组织中大量PMN浸润。PaO2降低是LIR致肺损伤进而导致气体交换功能明显障碍的直接表现,低氧进一步刺激外周化学感受器,反射性引起呼吸运动加深加快、CO2排出过多,从而使PaCO2明显降低。
肢体I-postC的实施,通过调动机体内源性的保护机制,下调了血浆和肺组织中ICAM-1、P-selectin及CD18水平,抑制PMN的局部黏附和释放炎性细胞因子,改善肺内微循环障碍,减轻了肺毛细血管内皮细胞的损伤,提高了肺换气的效率。光镜和电镜下肺组织的结构变化也从形态学角度支持I-postC对LIR后远位器官的保护作用。
| [1] | Yassin MM,Harkin DW,Barros AA,et al.Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction[J].World J Surg,2002,26(1):115-121. |
| [2] | 赵利军,门秀丽,李宏杰,等.缺血后适应减轻大鼠肢体缺血再灌注后的心肌损伤[J].中国病理生理杂志,2012,28(10):1892-1894,1900. |
| [3] | Yamamoto S,Tanabe M,Wakabayashi G,et al.The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine[J].J Surg Res,2001,99(1):134-141. |
| [4] | Men X,Han S,Gao J,et al.Taurine protects against lung damage following limb ischemia reperfusion in the rat by attenuating endoplasmic reticulum stress-induced apoptosis[J].Acta Orthop,2010,81(2):263-267. |
| [5] | Nemeth N,Lesznyak T,Brath E,et al.Changes in microcirculation after ischemic process in rat skeletal muscle[J].Microsurgery,2003,23(5):419-423. |
| [6] | Wang X,Kan S,Zhang B.Pathological changes in neuromuscular junction during ischemia-reperfusion in rat skeletal muscle[J].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi,2006,20(11):1103-1108. |
| [7] | Zambas NA,Karkos CD,Kambaroudis AG,et al.Protective effect of antithrombin Ⅲ against lung and myocardial injury in lower-limb ischemia-reperfusion syndrome[J].Ann Vasc Surg,2012,26(4):566-570. |
| [8] | Venna VR,Verma R,O'Keefe LM.et al.Inhibition of mitochondrial p53abolishes the detrimental effects of social isolation on ischemic brain injury[J].Stroke,2014,45(10):3101-3104. |
| [9] | Zhang J,Wu Y,Weng Z,et al.Glycyrrhizin protects brain against ischemia-reperfusion injury in mice through HMGB1-TLR4-IL-17A signaling pathway[J].Brain Res,2014,1582(5):176-186. |
| [10] | 苏建华,陈玉芳,丁新生,等.血必净对大鼠脑缺血再灌注损伤的保护作用及其机制[J].吉林大学学报:医学版,2015,41(5):951-955. |
| [11] | Zhang Y,Leng YF,Xue X,et al.Effects of penehyclidine hydrochloride in small intestinal damage caused by limb ischemia-reperfusion[J].World J Gastroenterol,2011,17(2):254-259. |
| [12] | Zhao W,Gan X,Su G,et al.The interaction between oxidative stress and mast cell activation plays a role in acute lung injuries induced by intestinal ischemia-reperfusion[J]. J Surg Res,2014,187(2):542-552. |
| [13] | Wu QL,Shen T,Ma H,et al.Sufentanil postconditioning protects themyocardium from ischemia-reperfusion via PI3K/Akt-GSK-3βpathway[J].J Surg Res,2012,178(2):563-570. |
| [14] | Moeini M,Nematbakhsh M,Fazilati M,et al.Protective role of recombinant human erythropoietin in kidney and lung injury following renal bilateral ischemia-reperfusion in rat model[J].Int J Prev Med,2013,4(6):648-655. |
 2016, Vol. 42
2016, Vol. 42


