扩展功能
文章信息
- 陈国兵, 吴谨准, 连珠兰, 占珠琴, 白海涛
- CHEN Guobing, WU Jinzhun, LIAN Zhulan, ZHAN Zhuqin, BAI Haitao
- 脂多糖对大鼠肺损伤及肺组织中AQP1和AQP5表达的影响
- Effects of lipopolysaccharide on lung injury and expressions of AQP1 and AQP5 in lung tissue of rats
- 吉林大学学报(医学版), 2016, 42(02): 250-254
- Journal of Jilin University (Medicine Edition), 2016, 42(02): 250-254
- 10.13481/j.1671-587x.20160212
-
文章历史
- 收稿日期: 2015-05-30
急性肺损伤(acute lung injury,ALI)是由严重感染和创伤等多种病理因素所致的弥漫性肺损伤性疾病,脂多糖(lipopolysaccharide,LPS)引起的脓毒症是造成ALI最常见的原因[1]。ALI主要病理基础是炎性细胞浸润和渗出性肺水肿导致急性呼吸衰竭,其发病机制尚未完全阐明[2]。水通道蛋白(aquaporins,AQPs)对水具有特异性转运功能,具有低抗肺水肿形成的作用[3, 4],研究[5, 6]表明:生理和病理情况下AQPs在肺泡细胞的水平衡调节上有重要作用。以往有关ALI的研究多集中于促进肺水肿的有害因素方面,如炎性介质、细胞因子等对肺血管内皮和肺上皮细胞的损伤作用[7],而有关对机体内调节肺水平衡的水通道蛋白1(AQP1)和水通道蛋白5(AQP5)的研究较少。本研究探讨大鼠经静脉注射LPS对ALI及肺组织中AQP1和AQP5表达的影响及其意义。
1 材料与方法 1.1 动物和主要试剂SPF级2月龄Wistar大鼠48只,雄性,平均体质量(285.7±15.0)g,购自上海斯莱克实验动物有限公司,动物许可证号:SCXK沪2003-0003。LPS (美国Sigma公司),肿瘤坏死因子α(tumor necrosis factor α,TNF-α)和巨噬细胞炎性蛋白1α(macrophage inflammatory protein-1α,MIP-1α)检测试剂盒(上海鑫乐生物公司),ECL化学发光试剂盒(美国Pierce公司),苏木素(福州迈新生物公司),Trizol和cDNA合成试剂盒和RT-qPCR试剂盒(日本Takara公司),RNA提取试剂盒和BCA蛋白质定量试剂盒(北京天根生化公司),PCR引物(美国Invitrogen公司),SDS和PVDF转印膜(美国PALL公司),兔抗大鼠AQP1抗体(美国Santa Cruz公司),兔抗小鼠AQP5抗体、兔抗大鼠β-Actin抗体和山羊抗兔多克隆二抗(美国Abcam公司)。
1.2 动物分组和模型制备48只大鼠随机分为对照组和LPS组,各组分为2、6、12和24h 4个时间点,每个时间点6只。LPS组大鼠经尾静脉注射LPS (5 mg·kg-1,浓度 5 g ·L-1),对照组大鼠注射等量生理盐水。观察LPS组大鼠呼吸频率、精神状态,当大鼠出现呼吸频率加快、躁动不安、紫绀时采取部分大鼠肺组织行HE染色、测定肺湿/干质量(wet/dry weight,W/D)比,检测血清TNF-α和MIP-1α水平,出现异常时提示大鼠ALI模型制备成功。
1.3 血清炎性因子和ALI相关指标测定 1.3.1 ELISA法检测大鼠血清TNF-α和MIP-1α水平大鼠腹腔注射3.5%的水合氯醛(10 mL·kg-1)麻醉,腹主动脉采血5mL注入生化管,3500 r·min-1 离心10 min,取上清液,按操作步骤进行TNF-α和MIP-1α水平测定。
1.3.2 HE染色病理学观察取右上小块肺组织经10%甲醛固定24h,常规石蜡包埋,5μm切片,苏木素-伊红(HE)染色,光镜下观察大鼠肺组织病理学表现。
1.3.3 W/D比值测定取右肺组织50~100mg,吸水纸吸干,立即称质量记作肺湿质量。然后置于80℃烤箱,48h后(质量不再减轻)称质量记为肺干质量,计算W/D比值,以此了解肺组织含水量。
1.3.4 支气管肺泡灌洗液(BALF)中蛋白水平和肺渗透指数测定采用BCA蛋白质定量试剂盒,用多功能酶标仪于562nm处检测其吸光度(A)值,绘出蛋白质标准曲线,根据A值计算血浆及BALF中蛋白水平,进而计算肺渗透指数。肺渗透指数=BALF蛋白水平 /血浆蛋白水平。
1.4 大鼠肺组织中AQP1和AQP5 蛋白及mRNA表达水平 1.4.1 Real-Time PCR检测大鼠肺组织中AQP1和AQP5 mRNA表达水平取大鼠肺组织50mg,采用TRizol法抽提总RNA,检测其浓度和纯度。以β-actin为内参,进行AQP1和AQP5 Real-Time PCR检测。AQP1(280 bp)上游引物序列:5'-TCACTTGGCCGAAATGACCTG-3',下游引物序列:5'-GTCCCACCCAGAAAATCCAGT-3';AQP5(151 bp)上游引物序列:5'-TCCAGGACCACACCAGAAAG-3',下游引物序列:5'-ATAAAATAGCACTCCGTGAGCC-3';β-actin(81 bp)上游引物序列:5'- ACTGCCGCATCCTCTTCCTC-3',下游引物序列:5'-GAACCGCTCATTGCCGATAGTG-3'。Real-Time PCR反应条件:95℃预变性30 s,95℃、5 s,58℃、20 s,72℃、20 s,共40个循环。AQP1和AQP5基因表达采用荧光定量PCR仪上的配套软件进行半定量分析。
1.4.2 Western blotting法检测AQP1及AQP5蛋白表达水平取大鼠同一部位肺组织样品约50mg,依次清洗、匀浆和裂解,常规提取组织蛋白,测定组织总蛋白浓度,取30μg蛋白质样品,加入等体积的2×SDS-PAGE样品缓冲液,沸水煮5min,进行SDS-PAGE蛋白电泳。其后转移至PVDF膜,按免疫印迹说明步骤操作,ECL发光、显影后用凝胶成像系统检测蛋白条带A值,以AQPs与β-actin蛋白的A值比值表示蛋白表达水平。
1.4.3 免疫组织化学法检测大鼠肺组织中AQP1和AQP5蛋白采用链霉亲和素-过氧化物酶复合物 (SABC)方法。5 μm切片脱蜡至水,3%过氧化氢(H2O2)灭活内源性过氧化物酶5~10 min,热修复抗原,血清封闭20 min,滴加AQPs一抗,4℃过夜,加山羊抗兔IgG(二抗),37℃、20 min,滴加SABC,37℃、20 min,每一次20min后均在磷酸盐缓冲液(PBS)中漂洗3次。二氨基联苯胺(DAB)显色,封片,电子显微镜观察、照相。染色结果判定:肺组织出现棕黄或棕褐色颗粒为阳性。
1.5 统计学分析采用SPSS 19.0软件包进行统计学分析。样本均来自正态分布总体,血清炎性因子及ALI相关指标以x±s表示,组间比较采用独立样本t检验,组内比较采用单因素方差分析。以P<0.05为差异有统计学意义。
2 结 果 2.1 各组大鼠血清炎性因子水平与对照组比较,在LPS注射后2、6和12h时间点LPS组大鼠血清TNF-α和MIP-1α水平均明显升高(P<0.05或P<0.01),至24h时间点逐渐恢复正常。见表 1。
| (n=6,x±s) | |||||
| Group | TNF-α | MIP-1α | W/D ratio | Protein in BALF | Permeability index(×10-3) |
| *P<0.05,** P<0.01 compared with control group. | |||||
| Control LPS 2 h 6 h z12 h 24 h | 70.26±2.62 78.66±6.64* 79.59±3.56** 79.69±5.96** 71.48±6.19 | 115±9 143±8* 157±7** 127±11* 115±16 | 4.22±0.35 5.32±0.71* 5.37±0.78* 5.72±0.88** 5.02±0.77* | 0.36±0.05 0.53±0.06* 0.55±0.11* 0.58±0.09** 0.50±0.03* | 3.50±0.23 5.56±0.35** 5.81±0.62* 5.95±0.23** 5.05±0.12* |
病理切片显示:LPS组2 h时间点大鼠出现肺泡和间质水肿及炎性细胞浸润,以6和12 h最为明显,至24h有所好转;对照组大鼠支气管、肺泡和血管结构正常。见图 1(插页三)。LPS组大鼠肺W/D比值、BALF中蛋白水平和肺渗透指数均高于对照组(P<0.05),12h时间点最明显。见表 1。

|
| A: Control group; B: LPS group (2 h). 图1 2组大鼠肺组织HE染色结果(×200) Fig.1 Results of HE staining of lung tissue of rats in two groups with immune cell(×200) |
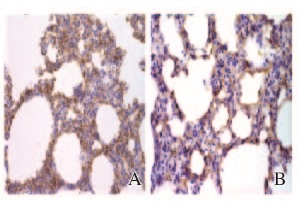
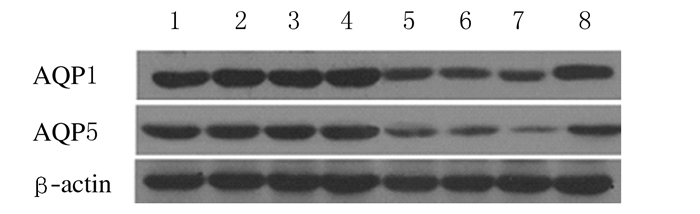
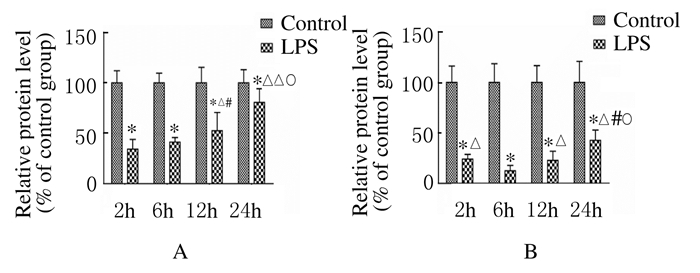
各组大鼠AQP1蛋白在肺间质出现阳性表达,AQP5蛋白在肺泡上皮细胞的顶膜面表达。见图 2(插页三)。与对照组比较,LPS组大鼠肺组织中AQP1和AQP5蛋白及mRNA表达水平明显下降(P<0.01),且随时间的延长逐渐下调,12h时间点表达水平最少,至24h时间点其表达水平有所升高。见图 3~5。
|
| 图2 AQP1(A)和AQP5(B)蛋白在大鼠肺组织中的表达(免疫组织化学,×400) Fig.2 Expressions of AQP1(A) and AQP5 (B) protein in lung tissue of rats ( Immunohistochemistry, ×400) |
|
| Lane 1-4:Control group at 2,6,12 and 24h;Lane 5-8:LPS group at 2,6,12 and 24h. 图3 Western blotting法检测大鼠肺组织中AQP1和AQP5蛋白表达 Fig.3 Expressions of AQP1 and AQP5 proteins in lung tissue of rats detected by Western blotting method |

|
| A:AQP1;*P<0.05,** P<0.01 vs control group;△P<0.05 vs 12 h in LPS group.B:AQP5; *P<0.01 vs control group;△P<0.01 vs 6 h in LPS group; #P<0.05 vs 2 h in LPS group. 图4 2组大鼠肺组织中AQP1和AQP5蛋白表达水平 Fig.4 Expression levels of AQP1 and AQP5 proteins in lung tissue of rats in two groups |
|
| A:AQP1;*P<0.01 vs control group;△P<0.05,△△P<0.01 vs 2 h;#P<0.01vs 6 h in LPS group; ○P<0.05 vs 12 h in LPS group;B:AQP5; *P<0.01 vs control group; △P<0.01 vs 6h in LPS group; #P<0.01 vs 2 h in LPS group; ○P<0.01 vs 12 h in LPS group. 图5 2组大鼠肺组织中AQP1和AQP5 mRNA表达水平 Fig.5 Expression levels of AQP1 and AQP5 mRNA in lung tissue of rats in two groups |
革兰阴性细菌感染产生内毒素,其主要成分LPS是引起脓毒症最常见的毒素[8],脓毒症后大量细胞因子释放,如TNF-α、MIP-1α和IL-6等,引起炎症级联效应[9],导致ALI。ALI以中性粒细胞聚集、肺泡上皮的完整性破坏为特点,导致肺泡-毛细血管基底膜受损,肺微血管通透性增高,引起肺泡和间质渗出性肺水肿[10, 11]。本实验中大鼠静脉注射LPS后出现烦躁、紫绀、呼吸加快;血清炎性因子TNF-α和MIP-1α水平明显升高;病理学显示肺泡上皮细胞出现肿胀、间质水肿,肺W/D比、BALF中蛋白水平和肺渗透指数均较对照组明显升高,说明大鼠注射LPS后导致ALI,随着时间的延长,肺组织损伤逐渐加重,12 h达高峰,24 h时血清炎性因子水平相对下降,肺损伤指标也逐渐改善。
ALI是一个组织弥散性肺泡炎和损伤后修复重建的复杂病理生理过程,其早期特征性改变是肺泡炎性水肿,继之出现肺间质增生和肺纤维化,最终导致呼吸功能的完全丧失[12, 13]。AQPs是一种对水具有特异性转运功能的细胞膜通道蛋白,在膜中以四聚体形式存在,每一单体形成一个功能性水通道,具有抵抗肺水肿形成的作用[14],目前在哺乳动物肺组织中有6种AQPs(AQP1、AQP3、AQP4、AQP5、AQP8和AQP9)表达并参与肺液体转运[15],研究[16, 17]表明:AQP1和AQP5参与了肺泡炎性水肿的形成,而AQP3和AQP4在肺水肿过程中无表达变化。本实验结果显示:AQP1在肺间质出现阳性表达,主要分布在肺血管内皮细胞和肺间质细胞膜,AQP5在肺泡上皮细胞顶膜和黏膜下腺泡细胞膜有表达。LPS组AQP1和AQP5蛋白及mRNA表达水平随时间的延长逐渐下调,与肺组织损伤相关指标的变化存在明显负相关关系,肺损伤越重,AQP1和AQP5表达水平越低。上述结果提示LPS造成的ALI和肺泡炎性水肿可能与炎性因子攻击导致肺组织AQP1和AQP5表达水平下调、进而降低了AQPs对水肿液体的清除能力有关。
ALI的发病机制尚未完全阐明,其病理基础为肺泡炎性水肿。如果能在肺损伤初期对肺组织水平衡进行干预,就有可能阻断ALI的发生、发展进程。AQPs在肺水转运以及水肿液清除方面的研究可能是未来的发展方向。
| [1] | Wang X,Zhang L,Duan W,et al. Anti-inflammatory effects of triptolide by inhibiting the NF-κB signalling pathway in LPS-induced acute lung injury in a murine model[J].Mol Med Rep,2014,10(1):447-452. |
| [2] | Zhan LY,Du L,Xia ZY,et al.Study of the expression of GABA(A) receptor in rats during acute lung injury caused by endotoxin[J].Genet Mol Res,2015,14(4):13312-13319. |
| [3] | Engelund MB,Madsen SS.The role of aquaporins in the kidney of euryhaline teleosts[J].Front Physiol,2011,2:51. |
| [4] | Nico B,Ribatti D.Role of aquaporins in cell migration and edema formation in human brain tumors[J].Exp Cell Res,2011,317(17):2391-2396. |
| [5] | Verkman AS.Role of aquaporins in lung liquid physiology[J].Respir Physiol Neurobiol,2007,159(3):324-330. |
| [6] | Jiao G,Li E,Yu R.Decreased expression of AQP1 and AQP5 in acute injured lung in rats[J].Chin Med J,2002,115(7):963-967. |
| [7] | Sapru A,Flori H,Quasney MW,et al.Pathobiology of acute respiratory distress syndrome[J].Pediatr Crit Care Med,2015,16(5 Suppl 1):S6-22. |
| [8] | Cross AS, Greenberg N, Billington M, et al.Phase 1 testing of detoxified LPS/group B meningococcal outer membrane protein vaccine with and without synthetic CPG 7909 adjuvant for the prevention and treatment of sepsis[J].Vaccine,2015,33(48):6719-6726. |
| [9] | 刘笑玎,原铭贞,王司仪,等.水通道蛋白1、3、4和5与急性肺损伤关系的研究进展[J].吉林大学学报:医学版,2014,40(5):1119-1122. |
| [10] | Ware LB,Herridge M.Acute lung injury[J].Semin Respir Crit Care Med,2013,34(4):439-440. |
| [11] | Zeng Z,Gong H,Li Y,et al.Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury[J].Exp Lung Res,2013,39(7):275-282. |
| [12] | Sharp C,Millar AB,Medford AR.Advances in understanding of the pathogenesis of acute respiratory distress syndrome[J].Respiration,2015,89(5):420-434. |
| [13] | Han S,Mallampalli RK.The acute respiratory distress syndrome:from mechanism to translation[J].J Immunol,2015,194(3):855-860. |
| [14] | Zhu C,Jiang Z,Bazer FW,et al.Aquaporins in the female reproductive system of mammals[J].Front Biosci (Landmark Ed),2015,20:838-871. |
| [15] | Zelenina M,Zelenin S,Aperia A.Water channels (aquaporins) and their role for postnatal adaptation[J].Pediatr Res,2005,57(5 Pt 2):47R-53R. |
| [16] | 陈国兵,许峰,卢仲毅,等.水通道蛋白在鼠肺发育过程中的变化及意义[J].中国病理生理杂志,2009,25(2):323-326. |
| [17] | Cher CD,Armugam A,Lachumanan R,et al.Pulmonary inflammation and edema induced by phospholipase A2:global gene analysis and effects on aquaporins and Na+/K+-ATPase[J].J Biol Chem,2003,278(33):31352-31360. |
 2016, Vol. 42
2016, Vol. 42
