扩展功能
文章信息
- 马小山, 孙婧, 曲丽梅, 曹赢坤, 刘文彬, 赵钥, 李蕴潜
- MA Xiaoshan, SUN Jing, QU Limei, CAO Yingkun, LIU Wenbin, ZHAO Yao, LI Yunqian
- 以视力下降为主要症状的老年鞍上区非典型脉络丛乳头状瘤1例报告及文献复习
- Atypical choroid plexus papilloma in suprasellar region of elderly with impaired vision as main symptom:A case report and literature review
- 吉林大学学报(医学版), 2020, 46(05): 1065-1069
- Journal of Jilin University (Medicine Edition), 2020, 46(05): 1065-1069
- 10.13481/j.1671-587x.20200527
-
文章历史
- 收稿日期: 2020-03-13
2. 吉林大学第一医院麻醉科, 吉林 长春 130021;
3. 吉林大学第一医院病理科, 吉林 长春 130021;
4. 吉林大学第一医院超声科, 吉林 长春 130031
2. Department of Anesthesiology, First Hospital, Jilin University, Changchun 130021, China;
3. Department of Pathology, First Hospital, Jilin University, Changchun 130021, China;
4. Department of Ultrasonography, First Hospital, Jilin University, Changchun 130031, China
非典型脉络丛乳头状瘤(atypical choroid plexus papilloma,ACPP)是一种介于脉络丛乳头状瘤(choroid plexus papilloma,CPP)和脉络丛乳头状癌(choroid plexus carcinoma,CPC)之间的一种交界性肿瘤,是2007年第4版世界卫生组织(WHO)中枢神经系统肿瘤分类中新增加的一种脉络丛肿瘤(choroid plexus tumors,CPTs)的亚型。ACPP约占CPTs的15%、颅内肿瘤的0.05%~0.10%。ACPP一般为低度恶性或交界性肿瘤[1-3]。ACPP常见于儿童和青少年,诊断的中位年龄为0.7岁,男女比例1:1。ACPP常发生在脑室,最常见于侧脑室三角区,其次是第三脑室和第四脑室[4]。发生在成年特别是老年患者鞍区或鞍上区的ACPP极为罕见。目前发生于成年患者且病变部位在鞍上区的病例尚未见报道。本文作者报道吉林大学第一医院神经外科收治的1例以视力下降为主要症状的鞍上区ACPP患者的诊疗经过,并进行相关文献复习,以期提高临床医生对该病的认识。
1 临床资料 1.1 一般资料患者,男性,61岁。因“持续性头痛1年余、双眼视力进行性下降2月余”于2019年6月20日收入吉林大学第一医院神经外科。入院查体:双眼视力下降,双侧颞侧视野缺损;无突眼、流泪、眼睑活动异常和眼球活动受限等症状;生命体征正常,四肢肌力、肌张力正常;其余各系统查体未见明显异常。
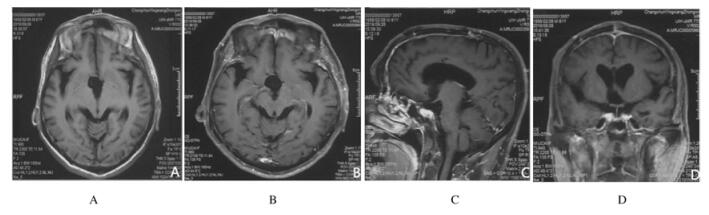
1.2 辅助检查部MRI平扫+增强扫描显示:鞍上区椭圆形肿块,大小为3.2 cm×2.7 cm×2.0 cm;T1WI显示不均匀低信号,T2WI显示不均匀高信号,增强扫描可见团块状高低混杂信号影,周边可见花环状强化(图 1)。实验室检查显示:0:00和8:00血清皮质醇含量降低(0:00,171.98 nmol·L-1;8:00,165.20 nmol·L-1;正常值为240.00~619.00 nmol·L-1)。

|
| A: Sagittal T2WI; B: Sagittal enhanced imaging; C: Coronal T2WI; D: Coronal enhanced imaging. 图 1 ACPP患者术前头部MRI影像 Fig. 1 Images of head MRI of ACPP patient before operation |
|
|
手术在全麻下进行,取患者发际线内右侧冠状切口,经额下入路行鞍上区肿物切除术。术中逐渐抬起额叶打开视交叉池,并解剖外侧裂池,逐步缓慢放出脑脊液,脑压明显下降,进一步抬起额颞叶。探查第二间隙,即可见肿瘤。肿瘤呈淡褐色及灰白色,形态呈分叶状,边界清楚,与周围结构无黏连,质地略韧,血运中等(图 2,见插页七)。在显微镜下将肿瘤全切。严密止血后以生理盐水冲洗术区,还纳颅骨修补骨窗,缝皮术终。术后病理检查:瘤内可见大量乳头状排列的肿瘤细胞,轻度异型,核分裂相1~2个/10个高倍视野。免疫组织化学:细胞增殖指数(Ki-67)(+3%~5%),波形蛋白(Vimentin)(+),胶质纤维酸性蛋白(glial fibrillary acidic protein,GFAP)(+),S-100(+),癌胚抗原(carcinoembryonic antigen,CEA)(局部散在+),甲状腺转录因子1(thyroid transcription factor 1,TTF-1)(+),EMA(局部散在+),广谱细胞角蛋白(CK-pan)(局部散在+),细胞角蛋白7(CK-7)(局部散在+),Syn(-),细胞角蛋白20(CK20)(-),甲状腺球蛋白(TG)(-),PR(-),CgA(-)(图 3,见插页七)。病理学诊断:结合免疫组织化学检查结果考虑为ACPP(WHO病理学标准:Ⅱ级)。
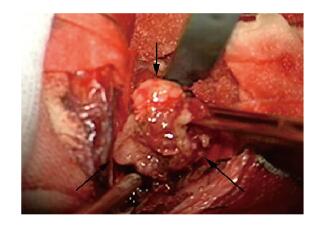
|
| The arrow showed the tumor. 图 2 ACPP患者术中肿瘤的大体形态 Fig. 2 Gross morphology of tumor in operation of ACPP patient |
|
|
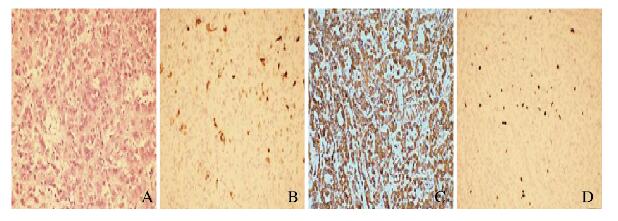
|
| A: HE staining; B: CK-7 staining; C: Vimentin staining; D: Ki-67 staining. 图 3 ACPP患者肿瘤组织HE染色和免疫组织化学染色结果(× 200) Fig. 3 Results of HE staining and immunohis tochemical staining of tumor tissue of ACPP patient(× 200) |
|
|
患者术后视力好转,视野基本恢复正常,无神经系统阳性体征。切口愈合良好,未接受放化疗等辅助治疗。术后3个月复查头颅MRI显示肿瘤全切除(图 4),未见肿瘤残余及复发。随访至今,未见肿瘤复发或转移。
|
| A: Axial T1WI; B: Axial enhanced imaging; C: Sagittal enhanced imaging; D: Coronal enhanced imaging. 图 4 ACPP患者术后3个月头部MRI影像 Fig. 4 Images of head MRI of ACPP patient at 3 months after operation |
|
|
CPTs是一种较少见的起源于脉络膜丛上皮的原发性中枢神经系统肿瘤,通常起源于脑室。每年CPTs的发生率为0.03/10万人,仅占颅内肿瘤的0.4%~0.8%,占儿童颅内肿瘤的1%~4%。2007年WHO根据组织病理学标准,将CPTs分为良性的CPP(WHO Ⅰ级)、处于良恶性之间的ACPP(WHO Ⅱ级)和CPC(WHO Ⅲ级)[1-4]。由于ACPP的罕见性,难以根据影像学资料做出较为准确的诊断,主要依靠组织病理学和免疫组织化学确诊。
多数ACPP原发于脑室内,其中侧脑室ACPP占总ACPP的53%,第四脑室占15%第三脑室占12%,桥脑小脑脚区占4%[5-22],而椎管内、脑实质中以及未被明确分类的ACPP各占3%[14-16, 20]。目前尚无研究者报道ACPP发生在鞍区及鞍上区的老年患者病例,本例患者根据症状、术中所见结合组织病理及免疫组织化学检测诊断为鞍上区ACPP。
与垂体瘤和颅咽管瘤等鞍区、鞍上区病损压迫下丘脑-垂体轴内分泌激素系统相似,患者术前和术后可出现血清皮质醇水平异常。本例ACPP患者血清皮质醇水平略低,但结合患者实际情况及现有的文献分析,该例患者术前无需使用皮质类固醇进行激素替代治疗[23-24]。
与其他类型CPTs相似,ACPP也来源于脉络丛上皮细胞,主要发生在脑室系统。脑室外CPTs的发生率相对较低[24]。影像学表现在鞍上区ACPP的诊断和鉴别诊断中具有重要意义。CPTs在T1WI上通常略微低信号或等信号,在T2WI时略微高信号或等信号,在增强MRI造影剂注入后成像中有中度或明显增强。ACPP在影像学上与CPP相似。肿瘤呈乳头状生长、分叶状改变且肿瘤内部囊变较多,囊壁及分隔均明显强化且与强化的脉络丛关系密切,为脉络丛肿瘤的典型影像学表现[12, 16]。本例患者的鞍区MRI表现符合CPTs。但由于本例患者年龄61岁,而且病变区域在鞍上区,根据MRI检查和临床表现将该患者术前初步诊断为胶质瘤。
病理诊断和免疫组织化学是诊断ACPP的金标准。在CPTs中,CPP的有丝分裂活性为每10个高倍镜视野下可见小于2个正在增殖的肿瘤细胞。不同于CPP完整而连续的基底膜以及非常低的有丝分裂活性,CPC是侵袭性强、恶性程度高的肿瘤,其特征为有至少4项恶性生物学行为,恶性生物学行为包括:细胞密度明显增加,乳头状结构模糊呈实性片状生长,较高的有丝分裂活性,细胞核多形化、核异型性明显、核质比增大、核分裂相明显增多以及肿瘤组织坏死。CPC的有丝分裂活性为每10个高倍镜视野下可见大于5个正在增殖的肿瘤细胞。镜下可见ACPP肿瘤组织中大量以血管为中心、周围由单层立方柱状上皮环绕的乳头状结构,与正常脉络丛组织非常相似。而ACPP的有丝分裂活性在两者之间,定义为每10个高倍镜视野下可见2~5个正在增殖的肿瘤细胞,但仅有1或2个CPC中的恶性组织学特点,不足以诊断为CPC时,可明确诊断为ACPP[1-2, 18-19, 22-23]。免疫组织化学检查,细胞角蛋白(cytokeratin)(CK7、CK-pan和CK-20)和vimentin几乎在所有CPP中均表达,可被看作是CPP的标志性蛋白。CK7(+)与CD20(-)的组合通常有助于区分原发性和转移性癌,后者通常显示不同的染色组合。Ki-67百分比与肿瘤级别存在直接联系,正常脉络丛组织接近零,同时CPP的Ki-67平均值为1.3%~4.5%,ACPP为5.8%~9.1%,而脉络丛为13.4%~20.3%[23]。本病例的组织病理学检查和免疫组织化学检查提示ACPP。
本文作者报道了1例罕见的61岁老年男性鞍上区ACPP病例。经幕上开颅手术将肿瘤全部切除,患者术后恢复良好。组织病理及免疫组织化学检测结果显示本病例符合ACPP病理学特点。本例患者肿瘤切除彻底,病理诊断恶性程度不高以及局部放疗对视交叉损伤较大,术后未给予放、化疗,建议定期复查鞍区MRI检查观察有无原位复发或转移。术后随访3个月无复发。目前,ACPP诊断仍面临许多挑战,特别是对于发生于不典型发病部位的病例。病理诊断是ACPP诊断的金标准。
| [1] |
WOLFF J A, SAJEDI M, BRANT R, et al. Choroid plexus tumours[J]. Br J Cancer, 2002, 87(10): 1086-1091. DOI:10.1038/sj.bjc.6600609 |
| [2] |
LOUIS D N, OHGAKI H, WIESTLER O D, et al. The 2007 WHO classification of tumours of the central nervous system[J]. Acta Neuropathol, 2007, 114(2): 97-109. DOI:10.1007/s00401-007-0243-4 |
| [3] |
DHILLON R S, WANG Y Y, MCKELVIE P A, et al. Progression of choroid plexus papilloma[J]. J Clin Neurosci, 2013, 20(12): 1775-1778. DOI:10.1016/j.jocn.2012.11.027 |
| [4] |
WREDE B, HASSELBLATT M, PETERS O, et al. Atypical choroid plexus papilloma:clinical experience in the CPT-SIOP-2000 study[J]. J Neuro Oncol, 2009, 95(3): 383-392. DOI:10.1007/s11060-009-9936-y |
| [5] |
BOSTRÖM A, BOSTRÖM J P, VON LEHE M, et al. Surgical treatment of choroid plexus tumors[J]. Acta Neurochir, 2011, 153(2): 371-376. DOI:10.1007/s00701-010-0828-x |
| [6] |
BRASSESCO M S, VALERA E T, BECKER A P, et al. Grade Ⅱ atypical choroid plexus papilloma with normal karyotype[J]. Childs Nerv Syst, 2009, 25(12): 1623-1626. DOI:10.1007/s00381-009-0938-z |
| [7] |
HOSMANN A, HINKER F, DORFER C, et al. Management of choroid plexus tumors:an institutional experience[J]. Acta Neurochir, 2019, 161(4): 745-754. DOI:10.1007/s00701-019-03832-5 |
| [8] |
IKOTA H, TANAKA Y, YOKOO H, et al. Clinicopathological and immunohistochemical study of 20 choroid plexus tumors:their histological diversity and the expression of markers useful for differentiation from metastatic cancer[J]. Brain Tumor Pathol, 2011, 28(3): 215-221. DOI:10.1007/s10014-011-0024-6 |
| [9] |
JAISWAL S, VIJ M, MEHROTRA A, et al. Choroid plexus tumors:A clinico-pathological and neuro-radiological study of 23 cases[J]. Asian J Neurosurg, 2013, 8(1): 29-35. DOI:10.4103/1793-5482.110277 |
| [10] |
JEIBMANN A, WREDE B, PETERS O, et al. Malignant progression in choroid plexus papillomas[J]. J Neurosurg, 2007, 107(3 Suppl): 199-202. DOI:10.3171/PED-07/09/199 |
| [11] |
KOH E J, WANG K C, PHI J H, et al. Clinical outcome of pediatric choroid plexus tumors:retrospective analysis from a single institute[J]. Childs Nerv Syst, 2014, 30(2): 217-225. DOI:10.1007/s00381-013-2223-4 |
| [12] |
LIN H, LENG X, QIN C H, et al. Choroid plexus tumours on MRI:similarities and distinctions in different grades[J]. Cancer Imaging, 2019, 19(1): 17. DOI:10.1186/s40644-019-0200-1 |
| [13] |
MA E M, WANG J, GUAN G F, et al. Microsurgical treatment of atypical choroid plexus papilloma in the fourth ventricle[J]. Acta Neurol Belg, 2016, 116(3): 413-414. DOI:10.1007/s13760-015-0551-8 |
| [14] |
MENON G, NAIR S N, BALDAWA S S, et al. Choroid plexus tumors:an institutional series of 25 patients[J]. Neurol India, 2010, 58(3): 429-435. DOI:10.4103/0028-3886.66455 |
| [15] |
QI Q C, NI S L, ZHOU X D, et al. Extraventricular intraparenchymal choroid plexus tumors in cerebral hemisphere:A series of 6 cases[J]. World Neurosurg, 2015, 84(6): 1660-1667. DOI:10.1016/j.wneu.2015.07.004 |
| [16] |
SHI Y Z, CHEN M Z, HUANG W, et al. Atypical choroid plexus papilloma:clinicopathological and neuroradiological features[J]. Acta Radiol Stock Swed, 2017, 58(8): 983-990. DOI:10.1177/0284185116676651 |
| [17] |
SIEGFRIED A, MORIN S, MUNZER C, et al. A French retrospective study on clinical outcome in 102 choroid plexus tumors in children[J]. J Neuro Oncol, 2017, 135(1): 151-160. DOI:10.1007/s11060-017-2561-2 |
| [18] |
TANAKA K, SASAYAMA T, NISHIHARA M, et al. Rapid regrowth of an atypical choroid plexus papilloma located in the cerebellopontine angle[J]. J Clin Neurosci, 2009, 16(1): 121-124. DOI:10.1016/j.jocn.2008.02.021 |
| [19] |
TENA-SUCK M L, SALINAS-LARA C, REMBAO-BOJ RQUEZ D, et al. Clinicopathologic and immunohistochemical study of choroid plexus tumors:single-institution experience in Mexican population[J]. J Neurol Oncol, 2010, 98(3): 357-365. DOI:10.1007/s11060-009-0080-5 |
| [20] |
YU H, YAO T L, SPOONER J, et al. Delayed occurrence of multiple spinal drop metastases from a posterior fossa choroid plexus papilloma.Case report[J]. J Neurosurg Spine, 2006, 4(6): 494-496. DOI:10.3171/spi.2006.4.6.494 |
| [21] |
ZHAO P, FENG Z C, Q I Q C, et al. Increased NG2 and SOX2 expression is associated with high-grade choroid plexus tumors[J]. Oncol Lett, 2017, 14(2): 1802-1806. DOI:10.3892/ol.2017.6326 |
| [22] |
ZHOU W J L, WANG X, PENG J Y, et al. Clinical features and prognostic risk factors of choroid plexus tumors in children[J]. Chin Med J, 2018, 131(24): 2938-2946. DOI:10.4103/0366-6999.247195 |
| [23] |
SAFAEE M, OH M C, BLOCH O, et al. Choroid plexus papillomas:advances in molecular biology and understanding of tumorigenesis[J]. Neurol Oncol, 2013, 15(3): 255-267. DOI:10.1093/neuonc/nos289 |
| [24] |
MCLAUGHLIN N, COHAN P, BARNETT P, et al. Early morning cortisol levels as predictors of short-term and long-term adrenal function after endonasal transsphenoidal surgery for pituitary adenomas and Rathke's cleft cysts[J]. World Neurosurg, 2013, 80(5): 569-575. DOI:10.1016/j.wneu.2012.07.034 |
 2020, Vol. 46
2020, Vol. 46


