扩展功能
文章信息
- 裴丹, 刘学
- PEI Dan, LIU Xue
- 抑制PDGFRα活化对小鼠脑损伤后胶质细胞增殖和瘢痕形成的影响
- Effect of inhibition of PDGFRα activation on glial cell proliferation and scar formation after brain injury in mice
- 吉林大学学报(医学版), 2020, 46(05): 1023-1028
- Journal of Jilin University (Medicine Edition), 2020, 46(05): 1023-1028
- 10.13481/j.1671-587x.20200520
-
文章历史
- 收稿日期: 2019-08-01
中枢神经系统(central nervous system,CNS)损伤往往伴随着血脑屏障(blood-brain barrier,BBB)破坏,浸润的粒细胞和CNS固有的胶质细胞过度增殖并分泌各种细胞因子和生长因子引发炎症反应[1]。在损伤的CNS各种轴突生长抑制因子,如硫酸软骨素蛋白多糖(chondroitinsul-phateproteoglycans,CSPGs),聚集在损伤周围形成瘢痕区[2]。抑制瘢痕形成可能是中枢神经损伤后促进轴突再生的新方法。与配体发生特异性结合的血小板衍生生长因子受体α(platelet-derived growth factor receptor α,PDGFRα),使受体二聚化暴露活性位点从而激发酪氨酸激酶的活性,激活受体的自体磷酸化[3]。作用于核内蛋白的转录因子,使细胞发生一系列的表型改变,促进细胞的生长、分裂[4]和增殖。小鼠脑损伤后在损伤周围会出现磷酸化的血小板衍生生长因子受体α(phosphorylated platelet-derived growth factor receptor α,p-PDGFRα)表达水平升高,而p-PDGFRα表达水平升高是否也与胶质细胞增殖及瘢痕形成有关尚未见相关报道。因此,本研究进一步探讨PDGFRα与细胞增殖及瘢痕形成的相关性,并观察PDGFRα抑制剂AG1296对细胞增殖和瘢痕形成的影响,以期为神经损伤后的再生修复治疗提供理论和实验依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器48只8周龄SPF级雄性BALB/c小鼠(中国医科大学实验动物部),体质量为25~35 g,动物生产许可证号:SYXK(辽)2013-0001。兔抗p-PDGFRα和兔抗PDGFRα(美国Santa Cruz公司),鼠抗GAPDH单克隆抗体(美国Proteintech公司),辣根酶标记的羊抗兔和羊抗鼠IgG(北京中杉金桥生物技术有限公司),兔抗胶质纤维酸性蛋白(glial fibrillary acidic protein,GFAP)、兔抗离子钙接头蛋白分子1(ionized calcium binding adaptor molecule 1,IBA-1)、兔抗Ⅳ型胶原蛋白(type Ⅳ collagen,Col Ⅳ)、兔抗NG2、兔抗纤连蛋白(fibronectin,FN)和兔抗CD45(英国Abcam公司)。小鼠脑立体定位仪(型号DW-2000,成都泰盟科技有限公司),高压灭菌器(型号VPV-5032,长春百奥技术有限公司),塑料薄膜封口机(型号SF300,温州兴业机械设备有限公司),转膜仪(美国Bio-Rad公司),恒温水浴箱(型号HHS21-6-S,上海跃进公司),水平摇床(型号WD-9405A,北京六一仪器厂)。
1.2 实验动物模型的制备及分组按照白丹等[5]的方法制备小鼠黑质纹状体通路损伤模型。用10%水合氯醛按体质量0.03 mL•g-1腹腔注射麻醉后固定在小鼠脑功能立体定位仪上。门齿栏设置成比耳屏线低3 mm。剪掉头皮顶部毛后消毒并在中央纵向切开,清除颅顶骨膜,在前囟点右后方1.5 mm处用牙钻缓慢钻出一个约1 mm×2 mm的长方形缺口,暴露大脑。将宽度为1.5 mm的切刀固定在直线型立体框架内,在右侧颅骨中线外侧0.5 mm,前囟点后1.5 mm处,距离大脑表面深度5.5 mm插入切刀。然后缓慢拔出刀片,止血缝合。将模型小鼠按照损伤后注入物质不同分为2组:黑质纹状体路部分切断+ PDGFRα抑制剂AG1296注入组(AG1296组),24只小鼠,手术后连续3 d内应用微量注射器沿损伤处注入AG1296,每日5 μL(5 mmol•L-1);二甲基亚砜(DMSO)对照组(DMSO组),24只小鼠,手术后连续3 d应用微量注射器注入同等剂量的DMSO。
1.3 取材及制片2组小鼠损伤后分别在1、4、7和14 d取脑,每次取6只小鼠。各组每个时间点取3只小鼠行心脏灌流冲洗,以损伤侧小鼠大脑半球的损伤部位为中心,取前后2 mm脑组织迅速放入-80℃冰箱保存备用。剩余3只小鼠行心脏灌流固定用于免疫组织化学染色,取脑组织4%多聚甲醛后固定过夜,逐级脱水,二甲苯透明,浸蜡,包埋。5 μm厚度水平连续切片,贴到多聚赖氨酸预处理的玻片烘干备用。
1.4 免疫组织化学染色检测各组小鼠脑组织中p-PDGFRα、GFAP、IBA-1、NG2、CD45、FN和ColⅣ的蛋白表达石蜡切片脱蜡水化,用0.01 mol•L-1的柠檬酸盐缓冲液微波沸腾修复,过氧化氢阻断,非免疫动物血清封闭,一抗p-PDGFRα(1︰100)、GFAP(1︰1 000)、IBA-1(1︰200)、NG2(1︰200)、CD45(1︰100)、FN(1︰200)和ColⅣ(1︰500),按比例稀释后滴至切片组织处4℃孵育过夜,生物素标记的二抗(S-P试剂盒C溶液),孵育10 min。链霉菌抗生素-过氧化物酶溶液(S-P试剂盒D溶液)孵育10 min后DAB溶液显色,显微镜下观察。自来水冲洗,梯度酒精脱水干燥,二甲苯透明,中性树胶封片。光学显微镜下观察免疫阳性产物的位置、分布、染色强弱及阳性细胞数,图像用NIS-Elements F系统拍片后以TIF文件保存。
1.5 Western blotting法检测各组小鼠脑组织中p-PDGFR-α、PDGFR-α、GFAP和IBA-1蛋白表达水平用0.9%生理盐水冲洗剪碎脑组织,添加组织质量4倍的含1% PMSF的RIPA裂解液后,超声粉碎机粉碎,4℃裂解30 min。4℃、12 000 r•min-1离心30 min,取上清液即为所得组织蛋白,BCA蛋白试剂盒进行蛋白定量,配置上样体系,上样体积10 µL,加入5×loading buffer 2 µL,剩余体积用PBS缓冲液补齐,100℃、5 min蛋白变性后‒80℃保存。配置8%和10%分离胶,微量注射器加样,浓缩胶内电泳电压设为80 V,分离胶内电泳电压设置为120 V。转膜后用含5%血清的TBST溶液37℃封闭1 h,加入一抗稀释液GAPDH(1︰10 000)、p-PDGFR-α(1︰200)、PDGFR-α(1︰200)、GFAP(1︰50 000)和IBA-1(1︰1 000),4℃冰箱内摇床上孵育过夜,洗膜后二抗工作液(1︰5 000)摇床孵育2 h。TBST漂洗后,ECL发光用Bio-Rad凝胶电泳图像分析仪进行采图。
1.6 统计学分析采用SPSS 19.0统计软件进行统计学分析。2组小鼠脑组织中p-PDGFRα、GFAP和IBA-1蛋白表达水平以x±s表示,所有实验重复3次。多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
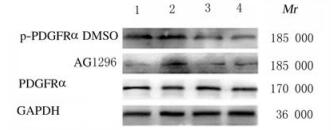
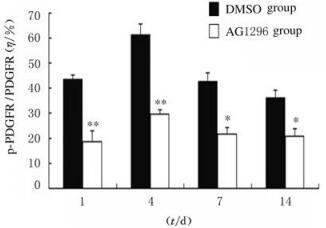
2 结果 2.1 各组小鼠脑损伤后脑组织中p-PDGFRα蛋白表达水平采用免疫组织化学染色和Western blotting法检测2组小鼠脑损伤1、4、7和14 d后p-PDGFRα蛋白表达。免疫组织化学染色检测结果显示:各时间点AG1296组小鼠脑组织中p-PDGFRα蛋白棕黄色阳性表达细胞数较DMSO组明显减少(图 1,见插页五)。Western blotting法检测结果显示:在相对分子质量为185 000标记处可见p-PDGFRα蛋白的免疫印迹条带(图 2);定量分析结果:各时间点AG1296组小鼠脑组织中p-PDGFRα蛋白表达水平明显低于DMSO组(P < 0.05或P < 0.01)(图 3)。

|
| A,B:1 d;C,D:4 d;E,F:7 d;G,H:14 d;A,C, E,G:DMSO group;B,D,F,H:AG1296 group. 图 1 2组小鼠脑损伤后脑组织中 p-PDGFRa 蛋白的表达 (免疫组织化学, Bar=100 μm) Fig. 1 Expressions of p-DGFRα protein in brain tissue of mice after injury in two groups (Immunohistochemistry,Bar=100 μm) |
|
|
|
| Lane 1: 1 d; Lane 2: 4 d; Lane 3: 7 d; Lane 4: 14 d. 图 2 Western blotting法检测2组小鼠脑损伤后脑组织中p-PDGFRα蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of p-pDGFRα protein in brain tissue of mice after injury in two groups detected by Western blotting method |
|
|
|
| *P < 0.05, **P < 0.05 compared with DMSO group. 图 3 2组小鼠脑损伤后不同时间点脑组织中p-PDGFRα蛋白表达水平 Fig. 3 Expression levels of p-PDGFRα protein in brain tissue of mice after brain injury |
|
|
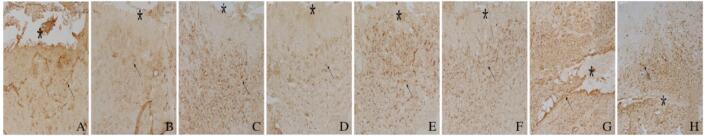
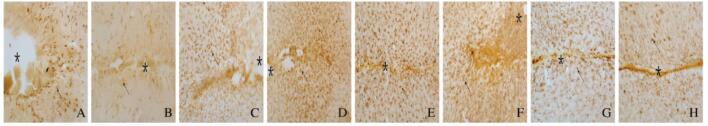
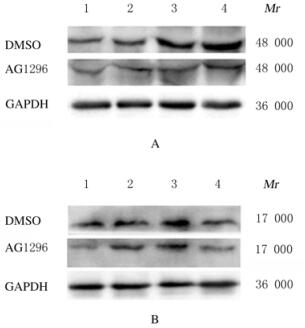
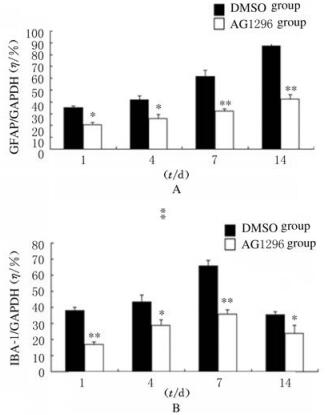
免疫组织化学染色检测结果显示:与DMSO组比较,AG1296组小鼠脑损伤后1、4、7和14 d损伤部位周围棕黄色星形胶质细胞数明显减少,并且细胞体积变小,突起变短(图 4,见插页六)。与DMSO组比较,AG1296组小鼠脑损伤部位周围小胶质细胞IBA-1阳性细胞数明显减少(图 5,见插页六)。Western blotting法检测结果显示:在相对分子质量为48 000和17 000标记处可见GFAP和IBA-1蛋白的免疫印迹条带(图 6);定量分析结果:AG1296组GFAP和IBA-1蛋白相对表达水平明显低于DMSO组(P < 0.05或P < 0.01)(图 7)。
|
| A,B:1 d;C,D:4 d;E,F:7 d;G,H:14 d;A,C, E,G:DMSO group;B,D,F,H:AG1296 group. 图 4 2组小脱脑拟伤后脑组织中 GFAP 蛋白表达 (免疫组织化学, Bar=200 μm) Fig. 4 Expressions of GFAP protein in brain tissue of mice in two groups ater injury (Immunohistochemistry,Bar=200 μm) |
|
|
|
| A,B:1 d;C,D:4 d;E,F:7 d;G,H:14 d;A,C, E,G:DMSO group;B,D,F,H:AG1296 group. 图 5 2组小鼠脑损伤后脑组织中 IBA-1 蛋白的表达 (免疫组织化学, Bar=200 μm) Fig. 5 Expressions of IBA-1 protein in brain tissue of mice after injury in two groups (Immunohistochemistry,Bar=200 μm) |
|
|
|
| A: GFAP; B: IBA-1; Lane 1: 1 d; Lane 2: 4 d; Lane 3: 7 d; Lane 4: 14 d. 图 6 Western blotting法检测2组小鼠脑损伤后脑组织中GFAP和IBA-1蛋白表达电泳图 Fig. 6 Elecrophoregram of expressions of GFAP and IBA-1 proteins in brain tissue of mice after injury in two groups detected by Western blotting method |
|
|
|
| A: GFAP; B: IBA-1 图 7 2组小鼠脑损伤后脑组织中GFAP和IBA-1蛋白表达水平 Fig. 7 Expression levels of GFAP and IBA-1 proteins in brain tissue of mice in two groups after injury |
|
|
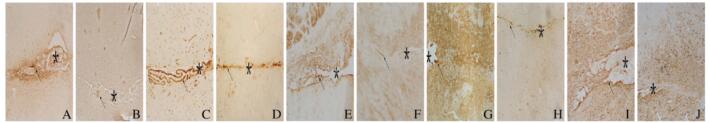
免疫组织化学染色结果显示:损伤14 d后,DMSO组小鼠脑组织中可见FN阳性细胞聚集在损伤中心部位(图 8A,见插页六),ColⅣ阳性细胞也密集地沉积在损伤的中心部位(图 8B,见插页六),表明纤维瘢痕形成在损伤中心部位;大量CD45阳性细胞聚集在损伤区周围(图 8C,见插页六),NG2阳性细胞即少突胶质前体细胞数在损伤位置大量增多(图 8D,见插页六),激活的星形胶质细胞(GFAP阳性细胞)在损伤周边明显增多并且其突起形成境界膜包围着纤维瘢痕(图 8E,见插页六)。AG1296组小鼠脑组织中,FN阳性细胞在损伤中心区明显减少(图 8F,见插页六),ColⅣ阳性细胞只有少量沉积在损伤的中心部位(图 8G,见插页六),表明纤维瘢痕形成明显减少,在纤维瘢痕区,损伤空洞消失,仅留一条缝隙;CD45阳性细胞少量存在于损伤区周围(图 8H,见插页六),NG2阳性细胞在损伤部位的周边明显减少(图 8I,见插页六),激活的星形胶质细胞(GFAP阳性细胞)虽然分布整个损伤区,但并未形成明显境界膜(图 8J,见插页六)。
|
| A-E: DMSO group; F-J: AG1296 group;A,F:FN;B,G: ColIN; C, H:CD45;D,1:NG2; E,J:GFAP. The area of asterisks indicated the injury sites, and the arrows refered to the positive cells. 图 8 2组小鼠脑损伤后14 d 脑组织中 FN 、ColIV 、CD45、NG2 和GFAP蛋白的表达 (免疫组织化学, Bar=200 μm) Fig. 8 Expressions of FN, ColIV ,CD45,NG2, and GFAP proteins in brain tissue of mice 14 d after injury in two groups (Immunohistochemistry,Bar=200 μm) |
|
|
成年的哺乳动物中枢神经创伤性损伤后横断的轴突几乎无再生能力,各种抑制因子在损伤周围阻止离断的轴突生长。本研究结果显示:DMSO组小鼠脑组织中FN阳性细胞聚集在损伤中心部位,ColⅣ阳性细胞也密集沉积在损伤的中心部位,表明纤维瘢痕形成在损伤中心部位,大量CD45阳性的粒细胞聚集在纤维瘢痕位置。研究者[6]发现:在脊髓损伤模型的纤维化组织中有巨噬细胞、淋巴细胞和成纤维细胞。研究[7-8]表明:瘢痕是脑损伤后主要阻止轴突再生的因素,脑损伤形成瘢痕表达各种潜在的轴突生长抑制因子,包括CSPGs、核因子E2相关因子2(NF-E2-related factor 2,Nrf2)[9-12]、黏蛋白-C和信号素3A,上述因子均可能是阻碍轴突再生的化学因素。LI等[13]和AHMED等[14]报道:在损伤的脊髓组织中加入ColⅣ的抑制剂DPY可以抑制纤维瘢痕的形成,促进轴突再生穿过损伤区,并能促进脊髓损伤后轴突再生和功能恢复。本研究结果显示:给予AG1296后14 d,小鼠脑损伤部位脑组织中FN、ColⅣ和CD45阳性细胞数较DMSO组减少,表明小分子抑制剂AG1296可以减少细胞增殖,进而减少纤维瘢痕的形成。本课题组将在后续实验中进一步验证抑制剂AG1296是否可以促进损伤后轴突再生和功能的恢复。
已有研究[15-18]表明:小鼠脑损伤后多种细胞因子刺激胶质细胞激活并增殖,如转化生长因子β(transforming growth factor β,TGF-β)、细胞外调节蛋白激酶(extracellular regulated protein kinase,ERK)、沉默调节蛋白1(recombinant sirtuin 1,Sirt1)和c-Jun氨基末端激酶(c-Jun N-terminal kinase,JNK)。通过调控Sirt1[19]抑制胶质细胞的激活可以促进神经元的恢复。本研究结果显示:小鼠脑损伤后激活PDGFRα,并且引起反应性的星形胶质细胞、小胶质细胞和NG2阳性的少突胶质前体细胞明显增多,胶质细胞的轴突最终封闭病变部位形成胶质界膜。这一结果导致即使神经纤维增生也无法穿透胶质界膜,进而影响损伤神经的修复;给予小分子PDGFRα抑制剂AG1296后,AG1296组小鼠脑组织中GFAP和IBA-1蛋白表达水平明显降低,星形胶质细胞和小胶质细胞增殖明显减少,尽管损伤周围的胶质细胞仍然很多,但胶质界膜基本消失,NG2蛋白免疫组织化学阳性表达的少突胶质前体细胞也明显减少,表明AG1296可以抑制PDGFRα的活化,减少胶质细胞反应和脑损伤后胶质瘢痕的形成及炎症细胞的扩散。因此,在脑损伤后期抑制PDGFRα信号的持续激活可能是减少细胞增殖,减少瘢痕形成,从而促进神经轴突再生的关键。从PDGFRα信号通路与瘢痕组织形成的相关性入手研究中枢神经损伤后的神经再生机制具有重要意义,而其作用的具体机制仍需进一步深入研究。
| [1] |
CORREALE J, VILLA A. The neuroprotective role of inflammation in nervous system injuries[J]. J Neurol, 2004, 251(11): 1304-1316. DOI:10.1007/s00415-004-0649-z |
| [2] |
YIU G, HE Z G. Glial inhibition of CNS axon regeneration[J]. Nat Rev Neurosci, 2006, 7(8): 617-627. DOI:10.1038/nrn1956 |
| [3] |
OSTENDORF T, EITNER F, FLOEGE J. The PDGF family in renal fibrosis[J]. Pediatr Nephrol, 2012, 27(7): 1041-1050. DOI:10.1007/s00467-011-1892-z |
| [4] |
HOU X, KUMAR A, LEE C, et al. PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets[J]. Proc Natl Acad Sci U S A, 2010, 107(27): 12216-12221. DOI:10.1073/pnas.1004143107 |
| [5] |
白丹, 李丹, 刘囡, 等. 降解纤维蛋白原可减少创伤性脑损伤后胶质瘢痕与纤维瘢痕的形成[J]. 解剖学报, 2014, 45(6): 729-734. |
| [6] |
KAWANO H, LI H P, SANGO K, et al. Inhibition of collagen synthesis overrides the age-related failure of regeneration of nigrostriatal dopaminergic axons[J]. J Neurosci Res, 2005, 80(2): 191-202. |
| [7] |
刘璐, 李丹, 刘囡, 等. 创伤性脑损伤后核因子E2相关因子2在星形胶质细胞中的表达[J]. 解剖学杂志, 2018, 41(5): 540-543. |
| [8] |
CHEN W, GUO Y J, YA NG, W J, et al. Phosphorylation of connexin 43 induced by traumatic brain injury promotes exosome release[J]. J Neurophysiol, 2018, 119(1): 305-311. DOI:10.1152/jn.00654.2017 |
| [9] |
LI X, WANG H D, GAO Y Y, et al. Protective effects of quercetin on mitochondrial biogenesis in experimental traumatic brain injury via the Nrf2 signaling pathway[J]. PLoS One, 2016, 11(10): e0164237. DOI:10.1371/journal.pone.0164237 |
| [10] |
ZHANG L, WANG H D, FAN Y W, et al. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways[J]. Sci Rep, 2017, 7: 46763. DOI:10.1038/srep46763 |
| [11] |
ZHANG L, WANG H D. Targeting the NF-E2-Related factor 2 pathway:a novel strategy for traumatic brain injury[J]. Mol Neurobiol, 2018, 55(2): 1773-1785. |
| [12] |
DING H, WANG H D, ZHU L, et al. Ursolic acid ameliorates early brain injury after experimental traumatic brain injury in mice by activating the Nrf2 pathway[J]. Neurochem Res, 2017, 42(2): 337-346. DOI:10.1007/s11064-016-2077-8 |
| [13] |
LI Y, LI D Q, RAISMAN G. Interaction of olfactory ensheathing cells with astrocytes may be the key to repair of tract injuries in the spinal cord:'the pathway hypothesis'[J]. J Neurocytol, 2005, 34(3-5): 343-351. DOI:10.1007/s11068-005-8361-1 |
| [14] |
AHMED Z, DENT R G, LEADBEATER W E, et al. Matrix metalloproteases:degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons[J]. Mol Cell Neurosci, 2005, 28(1): 64-78. DOI:10.1016/j.mcn.2004.08.013 |
| [15] |
LI D, TONG L, KAWANO H, et al. Protective effects of batroxobin on a nigrostriatal pathway injury in mice[J]. Brain Res Bull, 2016, 127(PtA): 195-201. |
| [16] |
LI D, TONG L, KAWANO H, et al. Regulation and role of ERK phosphorylation in glial cells following a nigrostriatal pathway injury[J]. Brain Res, 2016, 1648(Pt A): 90-100. |
| [17] |
LI D, LIU N, ZHAO H H, et al. Interactions between Sirt1 and MAPKs regulate astrocyte activation induced by brain injury in vitro and in vivo[J]. J Neuroinflammation, 2017, 14(1): 67. DOI:10.1186/s12974-017-0841-6 |
| [18] |
LI D, LIU N, ZHAO L, et al. Protective effect of resveratrol against nigrostriatal pathway injury in striatum via JNK pathway[J]. Brain Res, 2017, 1654(PtA): 1-8. |
| [19] |
ZHANG Z, LI D, XU L, et al. Sirt1 improves functional recovery by regulating autophagy of astrocyte and neuron after brain injury[J]. Brain Res Bull, 2019, 150: 42-49. DOI:10.1016/j.brainresbull.2019.05.005 |
 2020, Vol. 46
2020, Vol. 46


