扩展功能
文章信息
- 李小兵, 王旭, 吕瑛, 黄建成, 李红英, 王军, 张会军, 苏振宇
- LI Xiaobing, WANG Xu, LYU Ying, HUANG Jiancheng, LI Hongying, WANG Jun, ZHANG Huijun, SU Zhenyu
- 丹参多酚酸对心房颤动大鼠心肌组织中VCAM-1和ICAM-1水平及ERK1/2-NF-κB信号通路的影响
- Effects of salvianolic acid on levels of VCAM-1 and ICAM-1 in myocardium tissue and ERK1/2-NF-κB signaling pathway of atrial fibrillation rats
- 吉林大学学报(医学版), 2020, 46(05): 1004-1010
- Journal of Jilin University (Medicine Edition), 2020, 46(05): 1004-1010
- 10.13481/j.1671-587x.20200517
-
文章历史
- 收稿日期: 2019-12-14
2. 河北医科大学第一医院心脏外科, 河北 石家庄 050031
2. Department of Carcliac Surgery, First Hospital, Hebei Medical University, Shijiazhuang 050031, china
心房颤动(房颤)为临床上常见的心律失常之一,房颤患者常并发肾功能障碍和脑卒中等严重疾病,给患者、家庭和社会带来沉重负担。房颤病因尚不十分清楚,近年来,炎症反应与房颤的关系受到研究者的关注。研究[1-3]显示:房颤心肌组织中有多种炎症细胞浸润,表明炎症反应在房颤的疾病发生发展过程中发挥重要作用,抑制炎症反应可降低房颤的发病风险。丹参多酚酸为中药丹参水溶性活性成分,具有抑制血管损伤、抗心肌缺血、抗炎症反应和减轻钙超载等作用[4-5]。研究[6]显示:丹参多酚酸可通过降低心肌细胞重构过程改善房颤大鼠的心肌损伤程度,从而发挥治疗房颤的作用。丹参多酚酸还可以通过抑制炎症反应发挥对多种疾病的治疗作用,如其可通过抑制机体微炎症状态发挥对糖尿病肾病的治疗作用[7],通过抑制炎症反应改善不稳定型心绞痛的心功能[8]。但有关丹参多酚酸治疗房颤机制方面的研究尚未见相关报道,本研究通过分析丹参多酚酸对房颤大鼠心肌组织炎症反应和细胞外信号调节酶1/2-核转录因子-κB(extracellular signal-regulating enzymes 1/2-nuclear transcription factor-κB,ERK1/2-NF-κB)信号通路的影响,探讨丹参多酚酸是否通过抗炎作用发挥其对房颤的治疗作用。
1 材料与方法 1.1 实验动物、主要试剂和仪器清洁级健康雄性SD大鼠48只,12周龄,体质量200~250 g,购自北京华阜康生物科技股份有限公司,动物生产许可证号:SCXK(京)2014-001。注射用丹参多酚酸盐(规格:每瓶50 mg,批号:20190523,上海绿谷制药有限公司),苏木精-伊红(HE)染液(美国Sigma公司),RIPA裂解液、Masson试剂盒、免疫组织化学试剂盒、兔抗鼠基质金属蛋白酶2(matrix metalloproteinase-2,MMP-2)多克隆抗体、兔抗鼠基质金属蛋白酶9(matrix metalloproteinase-9,MMP-9)多克隆抗体、兔抗鼠血管细胞黏附分子1(vascular cell adhesion molecule-1,VCAM-1)多克隆抗体、兔抗鼠细胞间黏附分子1(intercellular adhesion molecule-1,ICAM-1)多克隆抗体、兔抗鼠ERK1/2多克隆抗体、兔抗鼠磷酸化ERK1/2(p-ERK1/2)多克隆抗体、兔抗鼠NF-κB多克隆抗体和兔抗鼠磷酸化NF-κB(p-NF-κB)多克隆抗体(美国Abcam公司)。TGL-16M高速离心机(湖南长沙湘仪仪器厂),EN61010-1水平电泳仪(美国Bio-Rad公司)。
1.2 实验动物模型建立、分组及给药方式将48只大鼠随机分为对照组、房颤组和丹参多酚酸组,每组16只。房颤模型建立:除对照组外,房颤组和丹参多酚酸组大鼠建立房颤模型[9],行刺激前先描记各大鼠心电图,然后舌下静脉注射氯化钙、乙酰胆碱混合液(1 mL•kg-1),再次描记大鼠心电图,待出现典型的房颤f波;隔日进行1次,共4周。造模成功标准:停药后心电图仍有典型的房颤波改变。建模成功后,丹参多酚酸组大鼠采用丹参多酚酸注射液(4 mg•kg-1•d-1)灌胃[10],对照组和房颤组大鼠灌胃给予等量生理盐水,每天1次,共4周。
1.3 实验动物取材治疗结束后处死大鼠,取心肌组织,将其分为2份:一份心肌组织经多聚甲醛固定72 h,常规脱水、石蜡包埋,用于HE染色、Masson染色和免疫组织化学染色;一份心肌组织用于Western blotting法测定。
1.4 HE染色观察大鼠心肌组织病理形态表现将石蜡切片烤片30 min,常规脱蜡,苏木精染色7 min,自来水冲洗,稀盐酸反应数秒,自来水冲洗,氨水返蓝,自来水冲洗,伊红染色,水洗,脱水透明,显微镜下观察大鼠心房组织病理形态表现。
1.5 Masson染色观察各组大鼠心肌纤维化情况将心肌组织石蜡切片脱蜡至水,苏木精染色10 min,盐酸乙醇分化,比布列猩红水-酸性品红-冰乙酸溶液染色2 min,磷钼酸-磷钨酸溶液染色15 min,苯胺蓝-冰乙酸溶液染色5 min,乙酸溶液浸洗5 min,常规脱水、透明、中性树胶封片,显微镜下观察大鼠心肌纤维化情况。
1.6 免疫组织化学染色检测各组大鼠心肌组织中MMP-2和MMP-9蛋白表达水平将心肌组织石蜡切片常规脱蜡至水,加入H2O2室温静置10 min,PBS缓冲液冲洗,山羊血清封闭液封闭20 min,加入一抗兔抗鼠MMP-2多克隆抗体和兔抗鼠MMP-9多克隆抗体孵育过夜,PBS缓冲液冲洗,加入二抗孵育1 h,PBS缓冲液冲洗,加入链霉素和过氧化物酶孵育30 min,PBS缓冲液冲洗,DAB显色剂显色,自来水冲洗,复染,脱水,透明,封片。采用BioMias 2000图像处理分析软件进行MMP-2和MMP-9蛋白阳性染色面积和积分光密度值测定。以积分光密度值代表蛋白表达水平。积分光密度=平均光密度×蛋白阳性染色面积。
1.7 Western blotting法测定各组大鼠心肌组织中VCAM-1、ICAM-1、ERK1/2、p-ERK1/2、NF-κB和p-NF-κB蛋白水平取心肌组织50 mg,加入裂解液提取总蛋白,煮沸表型,经电泳、转膜和封闭后,加入一抗:兔抗鼠VCAM-1多克隆抗体、兔抗鼠ICAM-1多克隆抗体、兔抗鼠ERK1/2多克隆抗体、兔抗鼠p-ERK1/2多克隆抗体、兔抗鼠NF-κB多克隆抗体和兔抗鼠p-NF-κB多克隆抗体,孵育过夜,加入二抗孵育2 h,采用Image lab software软件采集条带灰度值,以β-actin为内参。目标蛋白表达水平=目标蛋白条带灰度值/β-actin条带灰度值。
1.8 统计学分析采用SPSS 20.0统计软件进行统计学分析。各组大鼠心肌组织中MMP-2和MMP-9积分光密度值以及VCAM-1、ICAM-1、ERK1/2、p-ERK1/2、NF-κB和p-NF-κB蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用LSD-t检验,心肌组织中MMP-2和MMP-9蛋白表达水平与VCAM-1和ICAM-1蛋白表达水平相关性分析采用Pearson相关分析。以P< 0.05为差异有统计学意义。
2 结果 2.1 实验动物模型的建立停药后大鼠心电图仍有典型的房颤波改变,表明大鼠房颤模型建立成功。见图 1。
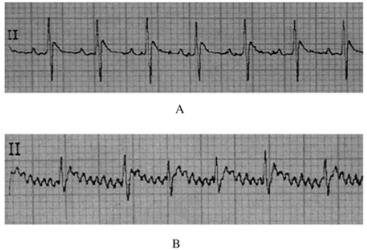
|
| A:ECG before modeling; B:ECG after modeling. 图 1 房颤模型大鼠造模前后ECG表现 Fig. 1 ECG performance of atrial fibrillation model rats before and after modeling |
|
|
对照组大鼠心肌细胞未见明显异常;房颤组大鼠心肌细胞排列紊乱,心肌组织间质增多,可见炎性细胞浸润;与房颤组比较,丹参多酚酸组大鼠心肌细胞排列尚正常,心肌组织间质稍多,炎性细胞浸润不明显。见图 2(插页四)。
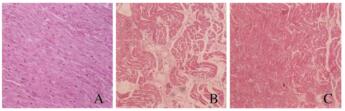
|
| A: Control group; B: Atrial fibrillation group; C: Salvianolic acid group. 图 2 各组大鼠心肌组织病理形态表现(HE, ×200) Fig. 2 Pathomorphology of myocardium tissue of rats in various groups (HE, ×200) |
|
|
对照组大鼠心肌间质胶原纤维正常;房颤组大鼠心肌间质胶原纤维明显增多;与房颤组比较,丹参多酚酸组大鼠心肌间质胶原纤维较房颤组明显减少。见图 3(插页五)。
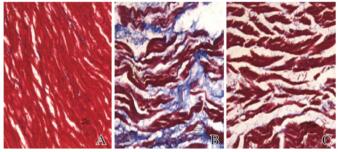
|
| A: Control group; B: Atrial fibrillation group; C: Salvianolic acid group. 图 3 各组大鼠心肌纤维化情况(Masson, ×400) Fig. 3 Myocardial fibrosis of rats in various groups (Masson, ×400) |
|
|
与对照组比较,房颤组和丹参多酚酸组大鼠心肌组织中MMP-2和MMP-9蛋白表达水平明显升高(P < 0.05);与房颤组比较,丹参多酚酸组大鼠心肌组织中MMP-2和MMP-9蛋白表达水平明显降低(P < 0.05)。见表 1和图 4(插页五)。
| (n=16, x±s) | |||||||||||||||||||||||||||||
| Group | MMP-2 | MMP-9 | |||||||||||||||||||||||||||
| Control | 0.12±0.03 | 0.08±0.02 | |||||||||||||||||||||||||||
| Atrial fibrillation | 0.87±0.06* | 0.51±0.04* | |||||||||||||||||||||||||||
| Salvianolic acid | 0.53±0.04*△ | 0.27±0.04*△ | |||||||||||||||||||||||||||
| F | 1 109.770 | 619.111 | |||||||||||||||||||||||||||
| P | <0.01 | <0.01 | |||||||||||||||||||||||||||
| *P < 0.05 compared with control group; △P < 0.05 compared with atrial fibrillation group. | |||||||||||||||||||||||||||||
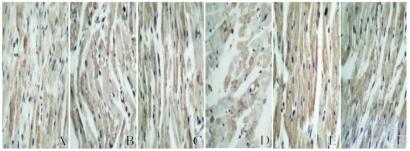
|
| A-C: MMP-2;D-F: MMP-9. A,D: Control group; B, E: Atrial fibrillation group; C,F: Salvianolic acid group. 图 4 各组大鼠心肌组织中MMP-2和MMP-9蛋白表达情况(免疫组织化学, ×400) Fig. 4 Expressions of MMP-2 and MMP-9 proteins in myocardium tissue of rats in various groups( Immunohistochemistry, ×400) |
|
|
与对照组比较,房颤组和丹参多酚酸组大鼠心肌组织中VCAM-1和ICAM-1蛋白表达水平升高(P < 0.05);与房颤组比较,丹参多酚酸组大鼠心肌组织中VCAM-1和ICAM-1蛋白表达水平降低(P < 0.05)。见图 5和表 2。
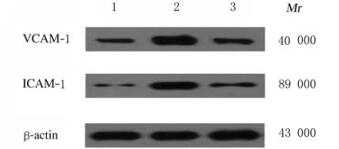
|
| Lane 1:Control group; Lane 2: Atrial fibrillation group; Lane 3: Salvianolic acid group. 图 5 各组大鼠心肌组织中VCAM-1和ICAM-1蛋白表达电泳图 Fig. 5 Electrophoregram of expressions of VCAM-1 and ICAM-1 proteins in myocardium tissue of rats in various groups |
|
|
| (n=16, x±s) | |||||||||||||||||||||||||||||
| Group | VCAM-1 | ICAM-1 | |||||||||||||||||||||||||||
| Control | 0.15±0.04 | 0.09±0.03 | |||||||||||||||||||||||||||
| Atrial fibrillation | 0.91±0.08* | 0.87±0.07* | |||||||||||||||||||||||||||
| Salvianolic acid | 0.27±0.06*△ | 0.21±0.05*△ | |||||||||||||||||||||||||||
| F | 690.759 | 1 020.145 | |||||||||||||||||||||||||||
| P | <0.01 | <0.01 | |||||||||||||||||||||||||||
| *P < 0.05 compared with control group; △P < 0.05 compared with atrial fibrillation group. | |||||||||||||||||||||||||||||
各组大鼠心肌组织中ERK1/2和NF-κB蛋白表达水平比较差异无统计学意义(P>0.05);与对照组比较,房颤组和丹参多酚酸组大鼠心肌组织中p-ERK1/2和p-NF-κB蛋白表达水平升高(P < 0.05);与房颤组比较,丹参多酚酸组大鼠心肌组织中p-ERK1/2和p-NF-κB蛋白表达水平降低(P < 0.05)。见表 3和图 6。
| (n=16, x±s) | |||||||||||||||||||||||||||||
| Group | ERK1/2 | p-ERK1/2 | NF-κB | p-NF-κB | |||||||||||||||||||||||||
| Control | 0.36±0.07 | 0.13±0.03 | 0.56±0.12 | 0.27±0.06 | |||||||||||||||||||||||||
| Atrial fibrillation | 0.35±0.06 | 0.32±0.06* | 0.56±0.11 | 0.94±0.08* | |||||||||||||||||||||||||
| Salvianolic acid | 0.37±0.06 | 0.21±0.05*△ | 0.58±0.13 | 0.44±0.07*△ | |||||||||||||||||||||||||
| F | 0.397 | 62.4 | 0.147 | 390.765 | |||||||||||||||||||||||||
| P | 0.675 | & #60;0.01 | 0.864 | & #60;0.01 | |||||||||||||||||||||||||
| *P < 0.05 compared with control group; △P < 0.05 compared with atrial fibrillation group. | |||||||||||||||||||||||||||||
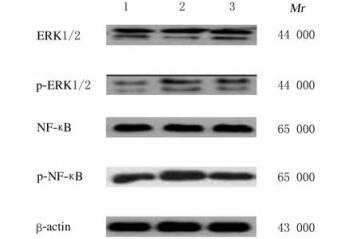
|
| Lane 1: Control group; Lane 2:Atrial fibrillation group; Lane 3: Salvianolic acid group. 图 6 各组大鼠心肌组织中ERK1/2、p-ERK1/2、NF-κB和p-NF-κB蛋白表达电泳图 Fig. 6 Electrophoregram of ERK1/2, p-ERK1/2, NF-κB, and p-NF-κB proteins in myocardium tissue of rats in various groups |
|
|
房颤组大鼠心肌组织中MMP-2和MMP-9蛋白表达水平与VCAM-1和ICAM-1蛋白表达水平呈正相关关系(P < 0.01)。见表 4。
| Index | VCAM-1 | ICAM-1 | |||
| r | P | r | P | ||
| MMP-2 | 0.435 | < 0.01 | 0.486 | < 0.01 | |
| MMP-9 | 0.512 | < 0.01 | 0.279 | < 0.01 | |
本研究中房颤组大鼠心肌组织中VCAM-1、ICAM-1、p-ERK1/2和p-NF-κB蛋白表达水平升高,表明房颤大鼠心肌组织存在炎症反应,且ERK1/2-NF-κB信号通路激活。房颤患者或动物模型中多种炎症细胞因子水平升高。VCAM-1和ICAM-1为膜表面糖蛋白,是介导细胞与细胞之间以及细胞与细胞外基质之间相互黏附、结合的黏附分子,VCAM-1和ICAM-1为体内重要的炎症因子。在多数单核细胞膜上均存在VCAM-1受体,VCAM-1主要发挥促进单个核细胞黏附作用;ICAM-1可介导细胞间识别,直接诱导白细胞聚集[11]。在白细胞介素1β(interleukin-1β,IL-1β)和肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)等多种炎症介质的作用下,VCAM-1和ICAM-1蛋白表达水平升高[12]。在房颤时大鼠心肌组织中VCAM-1和ICAM-1蛋白表达水平升高,其通过介导炎症反应引起心房组织结构改变,从而导致房颤的发生[13-14]。ERK1/2-NF-κB信号通路在炎症反应中发挥重要作用,在多种因素作用下,ERK1/2发生磷酸化,进而被激活,由细胞质转位到细胞核,进一步介导NF-κB磷酸化,p-NF-κB被进一步激活,促进炎性细胞活化,参与机体炎症反应[15-16]。NF-κB的靶基因众多,其靶基因(包括多种炎症细胞因子)均含有与NF-κB结合的位点,VCAM-1和ICAM-1序列中均含有NF-κB结合位点,故VCAM-1和ICAM-1的表达水平受NF-κB调控,NF-κB信号通路激活可促进VCAM-1和ICAM-1水平升高[17-18]。故激活的ERK1/2-NF-κB信号通路通过调控VCAM-1和ICAM-1炎症因子水平参与房颤的发生和维持。丹参多酚酸具有抗炎作用[19-20],本研究结果显示:丹参多酚酸治疗后房颤大鼠心肌组织中VCAM-1、ICAM-1、p-ERK1/2和p-NF-κB蛋白表达水平降低,表明丹参多酚酸可能通过抑制ERK1/2-NF-κB信号通路激活,从而下调VCAM-1和ICAM-1炎症因子水平,发挥对房颤的治疗作用。
本研究中房颤大鼠心肌组织中有炎症细胞浸润,可见大量胶原沉积,心肌组织中MMP-2和MMP-9蛋白表达水平升高。心肌纤维化为房颤心房结构重构的重要特征,心肌纤维化主要表现为心肌细胞外基质重构。心肌细胞外基质的主要成分为胶原蛋白,当受到异常刺激时心肌成纤维细胞大量合成和分泌胶原,导致细胞外基质中的胶原过量沉积,最终引起心肌纤维化,心肌纤维化对心房组织结构和功能造成破坏,使房颤更容易发生和维持[21]。MMPs在维持心肌细胞外基质的合成和降解中发挥重要作用,MMP-2和MMP-9为2种常用的MMPs,在心肌纤维化中发挥重要作用;正常情况下,MMP-2和MMP-9在细胞中以酶原形式存在,房颤早期细胞内存在钙超载,并可引起一系列病理生理变化,诱导MMP-2和MMP-9的分泌和活化,导致MMP-2和MMP-9活性增加,表达水平升高,引起正常的细胞外基质成分降解,同时合成不具有正常结构和功能的结缔组织和胶原蛋白,引起心肌组织重塑,导致房颤的发生发展[22]。故在房颤早期,细胞内钙超载诱导MMP-2和MMP-9分泌和活化,导致心肌组织不具有正常结构和功能的结缔组织和胶原蛋白增加,从而发生心肌重构,引起房颤的发生。MMPs与VCAM-1和ICAM-1分子共同参与多种疾病的发生发展,在房颤发病过程中MMPs与VCAM-1和ICAM-1相互作用参与房颤的发生发展[23-24]。本研究结果显示:房颤组大鼠心肌组织中MMP-2和MMP-9蛋白表达水平与VCAM-1和ICAM-1蛋白表达水平呈正相关关系;丹参多酚酸可降低房颤大鼠心肌组织中MMP-2和MMP-9蛋白表达水平。VCAM-1和ICAM-1作为炎症因子,通过影响MMP-2和MMP-9水平,引起房颤大鼠心肌纤维化,导致房颤发生;丹参多酚酸通过抑制炎症反应,降低炎症因子VCAM-1和ICAM-1水平,从而降低MMP-2和MMP-9水平,抑制心肌重构,阻止房颤的发生发展。
综上所述,丹参多酚酸治疗房颤可能是通过其抑制ERK1/2-NF-κB信号通路激活,下调其靶基因VCAM-1和ICAM-1炎症因子水平,影响MMP-2和MMP-9表达,从而抑制房颤心肌纤维化,发挥对房颤的治疗作用。
| [1] |
VAN WAGONER D R, CHUNG M K. Inflammation, inflammasome activation, and atrial fibrillation[J]. Circulation, 2018, 138(20): 2243-2246. DOI:10.1161/CIRCULATIONAHA.118.036143 |
| [2] |
YAO C X, VELEVA T, SCOTT L, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation[J]. Circulation, 2018, 138(20): 2227-2242. DOI:10.1161/CIRCULATIONAHA.118.035202 |
| [3] |
KORANTZOPOULOS P, LETSAS K P, TSE G, et al. Inflammation and atrial fibrillation:A comprehensive review[J]. J Arrhythm, 2018, 34(4): 394-401. DOI:10.1002/joa3.12077 |
| [4] |
WANG W, HU W. Salvianolic acid B recovers cognitive deficits and angiogenesis in a cerebral small vessel disease rat model via the STAT3/VEGF signaling pathway[J]. Mol Med Rep, 2018, 17(2): 3146-3151. |
| [5] |
SONG Y, LIU W H, DING Y, et al. Salvianolic acid A ameliorates renal ischemia/reperfusion injury by activating Akt/mTOR/4EBP1 signaling pathway[J]. Am J Physiol Ren Physiol, 2018, 315(2): F254-F262. DOI:10.1152/ajprenal.00508.2017 |
| [6] |
刘维琴, 李卫松, 马清华. 丹酚酸对房颤大鼠TNF-α, MMP-2/TIMP-2表达影响随机平行对照研究[J]. 实用中医内科杂志, 2013, 27(6): 83-85. |
| [7] |
何婉霞, 朱冠男. 高通量血液透析联合丹参多酚酸盐注射液对糖尿病肾病患者氧化应激及微炎症状态的影响[J]. 现代中西医结合杂志, 2019, 28(11): 1207-1210. |
| [8] |
李馨, 王文斌, 曹树军, 等. 丹参多酚酸盐辅助对不稳定型心绞痛患者TNF-α、hs-CRP、IL-1和心功能的影响[J]. 临床和实验医学杂志, 2018, 17(5): 499-503. |
| [9] |
陈春林, 巩甜甜, 汤依群, 等. SD大鼠房颤模型的建立[J]. 实验动物科学, 2009, 26(3): 1-4. |
| [10] |
刘维琴, 刘维蓉, 牟霞. 丹酚酸对房颤大鼠TNF-α, LTCCα1c蛋白表达影响随机平行对照研究[J]. 实用中医内科杂志, 2013, 27(2): 65-68. |
| [11] |
SONG F C, JI B, CHEN T. Cilostazol on the expression of ICAM-1, VCAM-1 and inflammatory factors in plasma in patients with thromboangiitis obliterans[J]. Exp Ther Med, 2018, 16(3): 2349-2354. |
| [12] |
HSU S F, LEE Y B, LEE Y C, et al. Dual specificity phosphatase DUSP6 promotes endothelial inflammation through inducible expression of ICAM-1[J]. FEBS J, 2018, 285(9): 1593-1610. DOI:10.1111/febs.14425 |
| [13] |
RUEDIGER C D, JOHN B, KUMAR S, et al. Influence of ethnic background on left atrial markers of inflammation, endothelial function and tissue remodelling[J]. Indian Pacing Electrophysiol J, 2018, 18(1): 1-5. DOI:10.1016/j.ipej.2017.08.002 |
| [14] |
WILLEIT K, PECHLANER R, WILLEIT P, et al. Association between vascular cell adhesion molecule 1 and atrial fibrillation[J]. JAMA Cardiol, 2017, 2(5): 516-523. DOI:10.1001/jamacardio.2017.0064 |
| [15] |
CHIN J P, CHEN C M, LEE T H, et al. Angiostrongylus cantonensis-conditioned culture medium induces myelin basic protein alterations via Erk1/2 and NF-κB activation in rat RSC96 schwann cells[J]. Chin J Physiol, 2018, 61(3): 137-143. DOI:10.4077/CJP.2018.BAG544 |
| [16] |
CHENG L J, CHEN Z H, WANG L H, et al. Propofol partially attenuates complete freund's adjuvant-induced neuroinflammation through inhibition of the ERK1/2/NF-κB pathway[J]. J Cell Biochem, 2019, 120(6): 9400-9408. DOI:10.1002/jcb.28215 |
| [17] |
ZHONG L, SIMARD M J, HUOT J. Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation[J]. FASEB J, 2018, 32(8): 4070-4084. DOI:10.1096/fj.201701536R |
| [18] |
OLIVARES S F, LANDAET R, ARANGUIZ P, et al. Heparan sulfate potentiates leukocyte adhesion on cardiac fibroblast by enhancing VCAM-1 and ICAM-1 expression[J]. Biochim Biophys Acta Mol Basis Dis, 2018, 1864(3): 831-842. DOI:10.1016/j.bbadis.2017.12.002 |
| [19] |
SUN Y, ZHAO D L, LIU Z X, et al. Inhibitory effect of salvianolic acid on inflammatory mediators of rats with collagen-induced rheumatoid arthritis[J]. Exp Ther Med, 2018, 16(5): 4037-4041. |
| [20] |
ZHANG H F, WANG Y L, GAO C, et al. Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats[J]. Acta Pharmacol Sin, 2018, 39(12): 1855-1864. DOI:10.1038/s41401-018-0026-6 |
| [21] |
ABE I, TESHIMA Y, KONDO H, et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation[J]. Heart Rhythm, 2018, 15(11): 1717-1727. DOI:10.1016/j.hrthm.2018.06.025 |
| [22] |
XUE Y M, DENG C Y, WEI W, et al. Macrophage migration inhibitory factor promotes cardiac fibroblast proliferation through the Src kinase signaling pathway[J]. Mol Med Rep, 2018, 17(2): 3425-3431. |
| [23] |
王栋, 白雪蕾, 吴环立. 急性期脑出血患者血清MMP-2 ICAM-1与IL-6 TNF-α水平和脑水肿的关系[J]. 中国实用神经疾病杂志, 2019, 22(12): 1336-1341. |
| [24] |
AHMED S, RIEGSECKER S, BEAMER M, et al. Largazole, a class I histone deacetylase inhibitor, enhances TNF-α-induced ICAM-1 and VCAM-1 expression in rheumatoid arthritis synovial fibroblasts[J]. Toxicol Appl Pharmacol, 2013, 270(2): 87-96. DOI:10.1016/j.taap.2013.04.014 |
 2020, Vol. 46
2020, Vol. 46


