扩展功能
文章信息
- 曾瑞霞, 张轶博
- ZENG Ruixia, ZHANG Yibo
- p62基因缺失对人脂肪间充质干细胞成脂的促进作用
- Promotion effect of p62 gene deletion on adipogenesis of human adipose-derived stromal cells
- 吉林大学学报(医学版), 2020, 46(05): 979-984
- Journal of Jilin University (Medicine Edition), 2020, 46(05): 979-984
- 10.13481/j.1671-587x.20200513
-
文章历史
- 收稿日期: 2019-11-02
2. 锦州医科大学基础医学院病原生物学教研室, 辽宁 锦州 121001
2. Department of Pathogeny Biology, School of Basic Medical Sciences, Jinzhou Medical University, Jinzhou 121001, China
肥胖发生的根本原因是脂肪细胞体积变大和数量增多,而后者很大程度上是由于脂肪分化异常所致[1-3]。脂肪间充质干细胞(adipose-derived stromal cells,ADSCs)作为脂肪细胞形成的源头,其增殖能力和分化潜能直接决定了成熟脂肪细胞的数量,影响脂肪组织的结构与构成。p62是自噬-溶酶体系统中重要的中介分子[4-5],p62基因敲除的小鼠发生肥胖和胰岛素抵抗,白色脂肪组织中脂肪积累增加,脂肪内脂质的合成增加[6]。自噬相关基因7(autophagy-related gene 7,Atg7)敲除的小鼠以及Atg7缺失的小鼠3T3-L1前脂肪细胞的研究显示:脂肪组织中总脂肪堆积量和脂滴数量减少,且脂滴体积缩小,小鼠3T3-L1前脂肪细胞分化的成熟脂肪细胞内脂滴数量减少,表明自噬被抑制后可降低甘油三酯积累,抑制脂肪细胞分化。线粒体自噬是一种选择性自噬,即自噬泡膜上有识别受损线粒体的特异受体,并对其针对性地进行降解,这对于维持线粒体正常的数量和功能非常重要。目前,关于脂肪分化的研究多以小鼠3T3-L1前脂肪细胞为模型,本研究选用人脂肪间充质干细胞(human adipose-derived stromal cells,hADSCs)作为研究对象进行脂肪分化研究,该细胞与小鼠3T3-L1细胞比较更接近人的生物学状态,对疾病的预防和治疗更具有指导意义。肥胖和高脂饮食有密切关联[7-8],而油酸(oleic acid,OA)是动物油和植物油中常见的游离脂肪酸,也是血液游离脂肪酸(free fatty acid,FFA)含量最多的成分,将其替代3-异丁基-1-甲基黄嘌呤(3-isobutyl-1-methylxanthine,IBMX)进行成脂诱导,可在一定程度上模拟人体的生理作用模式。p62是否通过调节脂肪细胞的分化而影响肥胖的发生尚未见文献报道,故本研究构建p62 shRNA慢病毒载体,探讨p62基因缺失对hADSCs成脂能力的影响以及与自噬之间的关系。脂肪分化过程中伴随着脂滴的形成及甘油三酯的合成,而线粒体是脂肪酸氧化的核心场所,因此在明确自噬在hADSCs成脂过程中的基本作用后,进一步探讨线粒体自噬与p62基因在hADSCs成脂中的作用及关系,为更深入阐明人脂肪分化的分子机制和寻找新药的作用靶点提供新思路。
1 资料与方法 1.1 研究对象、主要试剂和仪器脂肪组织取自3例大腿皮下脂肪抽吸术者(锦州医科大学附属第三医院整形外科),供者均为25~35岁的健康女性,并对实验知情同意。单丹磺酰戊二胺(monodansylcadaverine,MDC)(大连宝生物工程有限公司),细胞计数试剂盒(cell counting kit-8,CCK-8)(美国Invitrogen公司),Mitotraker Red探针(美国Invitrogen公司),一抗兔抗人β-actin、微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3,LC3)、CCAAT增强子结合蛋白α(CCAAT/enhancer binding protein alpha,CEBPα)、p62单克隆抗体和羊抗兔IgG-HRP二抗(美国Sigma公司)。DP71型正置荧光显微镜(日本Olympus公司),PRISM 7300型实时荧光定量PCR仪(美国ABI公司)。
1.2 p62基因的shRNA引物合成和慢病毒载体构建根据GenBank公布的人p62基因核苷酸序列(NM_001142299),人p62基因特异性shRNA靶序列(5'-GCAGATGAGGAAGATCGCCTT-3')的设计、合成以及慢病毒载体的构建由上海吉凯基因化学技术有限公司完成。
1.3 hADSCs的分离和培养将皮下脂肪抽吸液装入50 mL离心管,0.1%的Ⅰ型胶原酶进行消化,于37℃水浴箱作用1 h;加入等体积的完全培养基终止消化,1 500 r·min-1离心10 min,弃上清,留沉淀;将沉淀使用7 mL低糖DMEM进行吹打混匀,200目网过滤,收集滤液;1 000 r·min-1离心5 min,弃上清,留沉淀;L-DMEM吹打混匀沉淀,将其移入75 cm2的培养瓶中,放入5% CO2、37℃恒温细胞培养箱中进行常规培养。
1.4 OA诱导hADSCs的脂肪分化采用80 μmol·L-1 OA、10 mg·L-1胰岛素和1 μmol·L-1地塞米松在hADSCs成脂的全程进行持续诱导10 d,镜下观察成脂情况。
1.5 CCK-8实验绘制hADSCs生长曲线将对照组和p62基因缺失组hADSCs接种于96孔细胞培养板中,每孔1 × 103个细胞,并于接种后0~7 d检测细胞活性,每孔加入10 μL CCK-8溶液,于37℃、5% CO2孵箱中继续培养2 h,使用分光光度计在450 nm波长处测定吸光度(A)值,以A值为纵坐标,培养天数(d)为横坐标绘制细胞生长曲线。
1.6 Western blotting法检测hADSCs中p62、C/EBPα和LC3Ⅱ/LC3Ⅰ蛋白表达水平取第3代对数生长期hADSCs接种于6孔水平板中,待细胞达50%融合后加入重组慢病毒感染。24 h后PBS缓冲液冲洗3次,待细胞继续生长2 d,诱导10 d,进行尼罗红染色。于接种后10 d采用Western blotting法检测hADSCs中p62、C/EBPα和自噬相关蛋白LC3Ⅱ/LC3Ⅰ的表达水平。
1.7 MDC荧光染色检测自噬囊泡将P3代hADSCs接种到放有盖玻片的24孔细胞培养板上进行成脂诱导,3 d后进行染色观察。将细胞用PBS缓冲液清洗3次后,换为含MDC的培养基37℃孵育10 min,PBS缓冲液洗涤2次,取出盖玻片,用紫外光激发,于倒置荧光显微镜下观察和摄片。
1.8 Mitotracker Red荧光共定位检测hADSCs中线粒体自噬活性将P3代hADSCs接种到6孔细胞培养板上进行成脂诱导,3 d后加入200 nmol·L-1的Mitotracker Red进行染色15 min,PBS缓冲液冲洗3次,4%多聚甲醛固定15 min。加入2 mL 0.1%Trition X-100作用20 min;10%山羊血清封闭30 min;加入兔抗人LC3多克隆抗体,4℃过夜;加入Alexa Fluor鼠抗兔IgG二抗,避光,37℃孵育1 h。将细胞置于荧光显微镜下观察并采集图像,计数各组含脂滴细胞数。
1.9 统计学分析采用SPSS 17.0统计软件进行统计学分析。各组hADSCs细胞增殖活性,MDC染色阳性细胞数,各组hADSCs中p62、C/EBPα和LC3Ⅱ/LC3Ⅰ蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 hADSCs的形态表现分离培养的hADSCs接种24 h后可见细胞贴壁,呈短梭形。接种4 d后,细胞开始增殖,并充分伸展,呈多角形或梭形。传代后细胞生长速度加快,呈漩涡状排列(图 1,见插页三)。
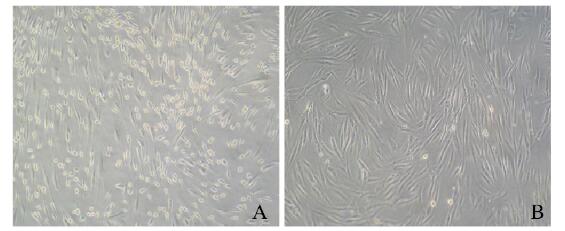
|
| A: Primary cells (4 d); B: Third generation. 图 1 显微镜下观察hADSCs的形态表现(×10) Fig. 1 Morphology of hADSCs observed under microscope (×10) |
|
|
选取80 μmol·L-1 OA联合地塞米松和胰岛素对hADSCs进行成脂诱导10 d,光镜下观察到细胞内有大量脂滴形成(图 2,见插页三)。
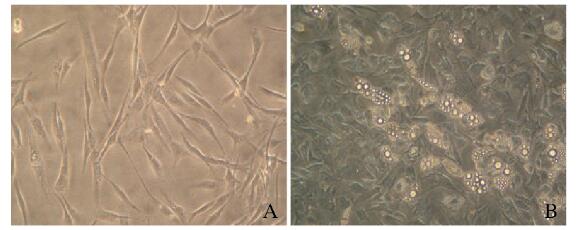
|
| A: Uninduced hADSCs; B: OA-induced apogenesis of hADSCs. 图 2 hADSCs成脂诱导第10天的形态表现(×20) Fig. 2 Morphology of hADSCs on 10th day after adipogenic induction (×20) |
|
|
将慢病毒shp62转染细胞24 h后,更换培养液继续培养,结果显示:细胞形态未发生明显变化。采用CCK-8比色法绘制生长曲线(图 3),结果显示:在培养7 d内,生长曲线呈“S”形,但p62基因缺失组生长曲线较低,细胞增殖缓慢;在培养5和6 d后p62基因缺失组细胞增殖活性较对照组明显降低(P < 0.05或P < 0.01)。
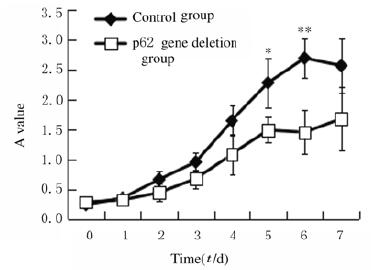
|
| *P < 0.05, **P < 0.01 compared with control group. 图 3 2组hADSCs的生长曲线 Fig. 3 Growth curves of hADSCs in two groups |
|
|
与对照组比较,p62基因缺失组hADSCs诱导脂肪分化至第3天时,细胞形态变圆,细胞内已出现微小的脂滴,而对照组诱导第5天才有脂滴出现;诱导分化至8~10 d,脂滴更丰富,细胞进一步变大变圆,尼罗红染色显示有丰富的脂滴,尼罗红脂滴染色阳性细胞数增多(图 4,见插页三)。Western blotting法检测结果显示:p62基因缺失组C/EBPα蛋白表达水平(1.40±0.10)较对照组(0.80±0.10)明显升高(P < 0.01)(图 5)。
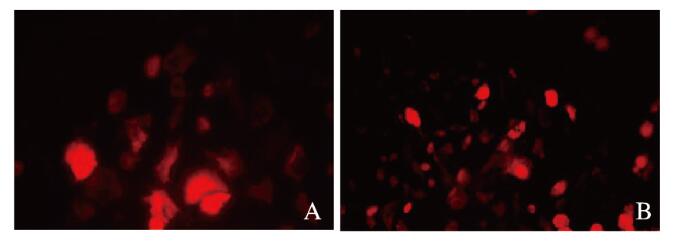
|
| A: Control group; B: p62 gene deletion group. 图 4 尼罗红染色检测2组hADSCs的成脂情况(×20) Fig. 4 Adipogenesis of hADSCs in two groups detected with Nile red staining(×20) |
|
|

|
| Lane 1:Control group; Lane 2: p62 gene deletion group. 图 5 Western blotting法检测2组hADSCs中C/EBPα蛋白表达电泳图 Fig. 5 Electrophoregram of expressions of C/EBPα protein in hADSCs in two groups detected by Western blotting method |
|
|
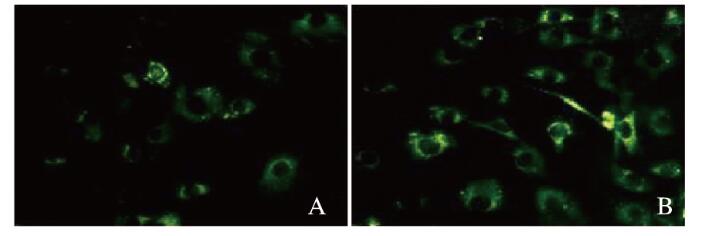
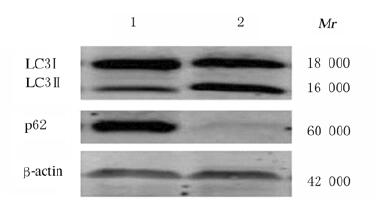
MDC染色结果显示:对照组MDC荧光呈弥散分布,p62基因缺失组为点状聚集,胞浆内荧光颗粒显著增多,荧光强度增加(图 6,见插页三),MDC染色阳性细胞数(26.13±1.24)较对照组(16.34±1.42)明显增加(P < 0.05)。Western blotting法检测结果显示:p62基因缺失组hADSCs中LC3Ⅱ/LC3Ⅰ蛋白表达水平(0.80±0.10)较对照组(0.33±0.15)明显升高(P < 0.01)(图 7)。
|
| A: Control group; B: p62 gene deletion group. 图 6 2组hADSCs的MDC染色结果(Bar=100 μm) Fig. 6 Results of MDC staining of hADSCs in two groups(Bar= 100 μm) |
|
|
|
| Lane 1:Control group; Lane 2: p62 gene deletion group. 图 7 Western blotting法检测2组hADSCs中LC3Ⅱ/LC3Ⅰ蛋白表达电泳图 Fig. 7 Electrophoregram of expressions of LC3Ⅱ/LC3Ⅰ proteins in hADSCs in two groups detected by Western blotting method |
|
|
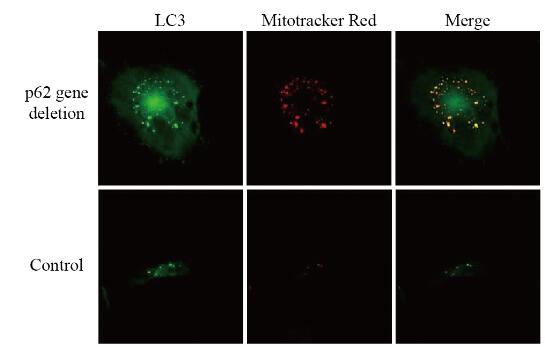
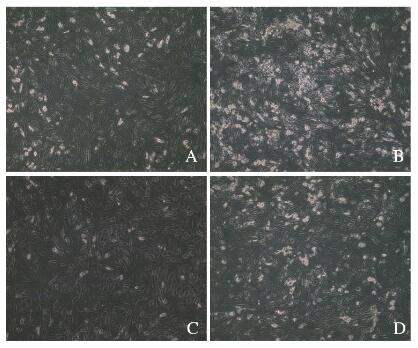
与对照组比较,在分化3 d,p62基因缺失hADSCs中LC3的点状化和颗粒化增多,与线粒体的共定位现象增加(图 8,见插页四)。在分化10 d,光镜下计数含脂滴细胞数(图 9,见插页四),对照组为(26.67±1.53)个,p62基因缺失组为(87.33±2.52)个,环孢菌素A(cyclsporin A,CsA)组为(14.67±2.52)个,p62基因缺失+ CsA组为(32.33±2.51)个。当p62基因缺失引起线粒体自噬的增加被CsA抑制后,其促进成脂的作用也被抑制,降低到接近对照组的水平。
|
| Green: LC3 immunofluorescent-labcled autophagosome; Red: Mitotracker Red labeled mitochondria; Yellow: Co-localization. 图 8 自噬体和线粒体在hADSCs成脂第3天的共定位(Mitotracker Red) Fig. 8 Colocalization of autophagosomes and mitochondria on third day after adipogenesis of hADSCs(Mitotracker Red) |
|
|
|
| A: Control group; B: p62 gene delction group; C: CsA group; D: p62 gene deletion+ CsA group. 图 9 CsA作用下hADSCs的成脂情况(油红O, × 10) Fig. 9 Adipogenesis of hADSCs after treated with CsA(Oil red O, ×10) |
|
|
p62基因敲除小鼠出现肥胖和胰岛素抵抗,基础脂水解变慢,并在其脂肪组织中发现脂滴数量增多,甘油三酯合成增加,脂肪细胞体积增大。本研究将慢病毒shp62转染至hADSCs中,内源p62基因沉默后,再进行成脂分化,结果显示:尼罗红染色阳性细胞数明显增多,调节成脂的关键分子C/EBPα的蛋白表达水平明显升高,表明p62基因缺失明显增强了hADSCs的成脂能力。干细胞的自我复制能力,也是干细胞维持“稳态”的一种体现,而p62基因缺失降低hADSCs的自我复制能力,在一定程度上表明干细胞维持稳态的能力下降,可能趋向分化。
鉴于p62作为自噬发生的重要中介物质,以及自噬在肥胖中的研究,本研究探讨p62基因与自噬在hADSCs成脂中的相互作用,MDC染色及LC3Ⅱ/LC3Ⅰ蛋白检测结果显示:p62基因的缺失在促进成脂过程中激活了自噬。研究[4]显示:如果p62基因缺失,与p62基因参与介导有关的自噬将降低;而本研究结果则显示:hADSCs中p62基因缺失导致自噬活性增强,与报道[4]结果相反,推测其可能原因:由于p62基因也可介导泛素蛋白酶体系统(UPS)对小分子蛋白的降解,因此p62基因缺失后,很多由p62基因介导的小分子物质不能被UPS清除,在细胞内大量聚集,后者可通过其他中介分子形成复合体,进而通过自噬进行降解,因此细胞的自噬活性增强。
研究[9-10]显示:线粒体在维持干细胞自我更新和定向分化中具有重要作用,当干细胞走向分化时,线粒体逐渐“成熟”,数量增加,功能增强。成脂过程中线粒体增加可能与以下2个因素有关:①细胞分化过程需要线粒体提供足够的ATP以保证细胞发生重建;②分化后成熟的脂肪细胞内有大量脂滴形成,线粒体的数量和功能对于脂滴及甘油三酯的合成有重要作用。Mitotracker Red探针对于线粒体的染色是亲脂的,不依赖于线粒体膜电位[11-12]。通过Mitotracker Red探针标记线粒体(红色)和LC3免疫荧光标记自噬体(绿色)不仅可以分别观察自噬体和线粒体[13-14],也可以在共聚焦显微镜下观察二者的共定位,以确定线粒体自噬的发生[15-17]。本研究中Mitotracker Red探针标记检测结果显示:p62基因缺失细胞中LC3的点状化和颗粒化增多,并观察到与线粒体的共定位现象增加,表明二者的复合体增多,提示p62基因缺失在hADSCs成脂中促进线粒体自噬。本研究使用CsA抑制线粒体自噬,当p62基因缺失引起线粒体自噬的增加被CsA抑制后(CsA + p62基因缺失),其促进成脂的作用也被抑制,表明p62基因缺失导致的成脂增加与线粒体自噬作用增强有关。关于p62基因缺失如何会增强线粒体自噬,可能与p62基因在机体中对线粒体作用的双面性有关:p62基因既可作为中介分子介导线粒体自噬发生,也可稳定线粒体的形态使其免受损伤而抑制线粒体自噬的发生[18-20]。
综上所述,在hADSCs脂肪分化过程中,p62基因缺失不仅导致自噬流增加,而且使得自噬流的分布不均:线粒体导向的自噬流增多,保证了hADSCs分化中所需大量线粒体的更新,使分化进一步延续,脂质合成逐渐增加,最终使得成脂增加。
| [1] |
ALSHAMMARI G M, BALAKRISHNAN A. Pumpkin (Cucurbita ficifolia Bouché) extract attenuate the adipogenesis in human mesenchymal stem cells by controlling adipogenic gene expression[J]. Saudi J Biol Sci, 2019, 26(4): 744-751. DOI:10.1016/j.sjbs.2018.10.002 |
| [2] |
SU Y, DENBEIGH J M, CAMILLERI E T, et al. Extracellular matrix protein production in human adipose-derived mesenchymal stem cells on three-dimensional polycaprolactone (PCL) scaffolds responds to GDF5 or FGF2[J]. Gene Rep, 2018, 10: 149-156. DOI:10.1016/j.genrep.2017.12.004 |
| [3] |
LU Y, CHEN J Y, XIAN T, et al. Epigallocatechin-3-gallate suppresses differentiation of adipocytes via regulating the phosphorylation of FOXO1 mediated by PI3K-AKT signaling in 3T3-L1 cells[J]. Oncotarget, 2018, 9(7): 7411-7423. DOI:10.18632/oncotarget.23590 |
| [4] |
TSUCHIYA M, ISOGAI S, TANIGUCHI H, et al. Selective autophagic receptor p62 regulates the abundance of transcriptional coregulator ARIP4 during nutrient starvation[J]. Sci Rep, 2015, 5: 14498. DOI:10.1038/srep14498 |
| [5] |
ZHOU L, WANG H F, REN H G, et al. Bcl-2 decreases the affinity of SQSTM1/p62 to poly-ubiquitin chains and suppresses the aggregation of misfolded protein in neurodegenerative disease[J]. Mol Neurobiol, 2015, 52(3): 1180-1189. |
| [6] |
MOSCAT J, DIAZ-MECO M T. Feedback on fat:p62-mTORC1-autophagy connections[J]. Cell, 2011, 147(4): 724-727. DOI:10.1016/j.cell.2011.10.021 |
| [7] |
ADAMSKA E, OSTROWSKA L, GOŚCIK J, et al. Intake of meals containing high levels of carbohydrates or high levels of unsaturated fatty acids induces postprandial dysmetabolism in young overweight/obese men[J]. Biomed Res Int, 2015, 2015: 147196. |
| [8] |
ARNER P, RYDÉN M. Fatty acids, obesity and insulin resistance[J]. Obes Facts, 2015, 8(2): 147-155. DOI:10.1159/000381224 |
| [9] |
KIM E, HWANG S U, YOO H, et al. Putative embryonic stem cells derived from porcine cloned blastocysts using induced pluripotent stem cells as donors[J]. Theriogenology, 2016, 85(4): 601-616. DOI:10.1016/j.theriogenology.2015.09.051 |
| [10] |
WANG L, YE X Y, ZHAO Q, et al. Drp1 is dispensable for mitochondria biogenesis in induction to pluripotency but required for differentiation of embryonic stem cells[J]. Stem Cells Dev, 2014, 23(20): 2422-2434. DOI:10.1089/scd.2014.0059 |
| [11] |
GHARAGOZLOO M, RAFIEE A, CHEN D W, et al. A flow cytometric approach to study the mechanism of gene delivery to cells by gemini-lipid nanoparticles:an implication for cell membrane nanoporation[J]. J Nanobiotechnol, 2015, 13: 62. DOI:10.1186/s12951-015-0125-1 |
| [12] |
ZHANG G, SUN Y M, HE X Q, et al. Red-emitting mitochondrial probe with ultrahigh signal-to-noise ratio enables high-fidelity fluorescent images in two-photon microscopy[J]. Anal Chem, 2015, 87(24): 12088-12095. DOI:10.1021/acs.analchem.5b02807 |
| [13] |
REAL E, RODRIGUES L, CABAL G G, et al. Plasmodium UIS3 sequesters host LC3 to avoid elimination by autophagy in hepatocytes[J]. Nat Microbiol, 2018, 3(1): 17-25. DOI:10.1038/s41564-017-0054-x |
| [14] |
HAMURCU Z, DELIBAŞI N, GEÇENE S, et al. Targeting LC3 and Beclin-1 autophagy genes suppresses proliferation, survival, migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin beta1/Src signaling in triple negative breast cancer cells[J]. J Cancer Res Clin Oncol, 2018, 144(3): 415-430. DOI:10.1007/s00432-017-2557-5 |
| [15] |
WANG H, JIANG T Y, LI W, et al. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer's disease[J]. Toxicol Lett, 2018, 282: 100-108. DOI:10.1016/j.toxlet.2017.10.021 |
| [16] |
INDIRA D, VARADARAJAN S N, SUBHASINGH LUPITHA S, et al. Strategies for imaging mitophagy in high-resolution and high-throughput[J]. Eur J Cell Biol, 2018, 97(1): 1-14. DOI:10.1016/j.ejcb.2017.10.003 |
| [17] |
BIEL T G, RAO V A. Mitochondrial dysfunction activates lysosomal-dependent mitophagy selectively in cancer cells[J]. Oncotarget, 2018, 9(1): 995-1011. DOI:10.18632/oncotarget.23171 |
| [18] |
沈君颜, 王浩, 刘海亮. microRNA调控间充质干细胞成骨分化的研究进展[J]. 同济大学学报(医学版), 2020, 41(1): 123-129, 135. |
| [19] |
LAM H C, BAGLINI C V, LOPE A L, et al. p62/SQSTM1 cooperates with hyperactive mTORC1 to regulate glutathione production, maintain mitochondrial integrity, and promote tumorigenesis[J]. Cancer Res, 2017, 77(12): 3255-3267. DOI:10.1158/0008-5472.CAN-16-2458 |
| [20] |
HA S, JEONG S H, YI K, et al. Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells[J]. J Biol Chem, 2017, 292(33): 13795-13808. DOI:10.1074/jbc.M117.780874 |
 2020, Vol. 46
2020, Vol. 46


