扩展功能
文章信息
- 陈家显, 刘先霞, 陈跃武, 陈磊, 张远生, 陈劲松
- CHEN Jiaxian, LIU Xianxia, CHEN Yuewu, CHEN Lei, ZHANG Yuansheng, CHEN Jinsong
- 芪参益气滴丸对慢性心力衰竭大鼠心肌细胞凋亡的抑制作用及其机制
- Inhibitory effect of Qisheng-Yiqi Dropping Pill on apoptosis of myocardial cells in rats with chronic heart failure and its mechanism
- 吉林大学学报(医学版), 2020, 46(05): 972-978
- Journal of Jilin University (Medicine Edition), 2020, 46(05): 972-978
- 10.13481/j.1671-587x.20200512
-
文章历史
- 收稿日期: 2020-01-11
2. 南华大学附属第二医院心血管内科, 湖南 衡阳 421001
2. Department of Cardiovascular Medicine, Second Affiliated Hospital, South China University, Hengyang 421001, China
慢性心力衰竭(chronic heart failure,CHF)是一种常见的心血管疾病,也是各种心脏疾病的终末期表现,近年来其发病率呈上升趋势,且因其具有高发病率和高死亡率的特点已引起研究者高度关注[1]。目前CHF的治疗仍以药物治疗为主,西药控制心力衰竭进展及改善症状有良好的效果,但其局限于对症处理,且药物不良反应明显。中医药治疗心血管疾病已体现出其独特优势,近年来的研究[2-3]表明:中医药治疗心血管疾病疗效确切,安全性高,且具有多靶点、多层次和多环节等优势。芪参益气滴丸(Qishen-Yiqi Dropping Pill,QSYQ)是治疗气虚血瘀型胸痹的中药制剂,主要由黄芪、丹参、三七和降香组成,具有益气活血的功效,临床广泛地应用于冠心病及心力衰竭的治疗,并取得了良好效果[4-5],但其作用机制尚未明确。本研究通过观察CHF大鼠心脏超声表现、心肌细胞形态、氧化应激水平及细胞凋亡蛋白的表达,探讨QSYQ对心力衰竭发生时氧化应激水平和心肌细胞凋亡的影响,阐明其在CHF发生发展过程中的保护作用及其可能作用机制。
1 材料与方法 1.1 实验动物、主要试剂和仪器无特定病原体(SPF)级雄性SD大鼠60只,鼠龄6~7周,体质量(220±20)g,由湖北省动物实验中心提供,动物生产许可证号:SCXK(鄂)2018-0027。大鼠饲养条件:温度24℃,湿度50%,通风良好,明暗光照交替12 h,标准饲料喂养,自由进食及饮水。适应环境1周,术前禁食24 h,不禁水。QSYQ(批号150906)购于天津天士力制药集团股份有限公司,卡托普利(批号H31022986)购于中美上海施贵宝制药有限公司,苏木素、脱氧核糖核苷酸末端转移酶介导的缺口末端标记法(TUNEL)细胞凋亡检测试剂盒和活性氧(ROS)检测试剂盒购于上海碧云天生物技术有限公司,乳酸脱氢酶(LDH)、超氧化物歧化酶(SOD)和丙二醛(MDA)试剂盒购于南京建成生物工程研究所,活化半胱氨酸天冬氨酸蛋白酶3(Cleaved-Caspase-3)、B淋巴细胞瘤-2(Bcl-2)、Bcl-2相关X蛋白(Bax)、核因子E2相关因子2(Nrf2)和血红素氧化酶1(HO-1)抗体购于美国Cell Signaling Technology公司。ACUSON X300超声心动图仪购于德国西门子公司,RX50M正置显微镜和摊片机购于德国徕克显微系统有限公司,UniCel DxC600全自动生化分析仪购于美国Beckman Coulter公司,Tanon-3500(R)全自动数码凝胶图像分析系统购于上海天能科技有限公司,Varioskan LUX酶标仪购于美国Thermo Fisher Science公司。
1.2 实验动物模型制备和分组60只SPF级雄性SD大鼠,适应性饲养1周后,随机分为假手术组、模型组、阳性药对照组(6.75 mg·kg-1·d-1卡托普利)[6-7]、低剂量(135 mg·kg-1·d-1)QSYQ组和高剂量(270 mg·kg-1·d-1)QSYQ组,每组12只。3%戊巴比妥钠腹腔注射麻醉大鼠,采用冠状动脉前降支结扎法建立CHF模型(假手术组不结扎冠状动脉)[8],造模结束后利用超声心动图仪检测大鼠心功能,左室射血分数(ejection raction,EF)≤50%视为CHF造模成功[9-10],术后24 h灌胃给药,连续4周,其中假手术组和模型组大鼠每天给予其他3组等量生理盐水灌胃。
1.3 超声检查各组大鼠灌胃4周后进行心脏超声检测,记录左室收缩末期内径(left ventricular end-systolic diameter,LVSD)、左室舒张末期内径(left ventricular end-diastolic diameter,LVDD)、EF和左室短轴缩短率(left ventricular short-axis shortening rate,FS)。
1.4 TUNEL法检测大鼠心肌组织细胞凋亡率3%戊巴比妥钠腹腔注射麻醉大鼠,取左室心肌组织,洗净后用10%甲醛固定24 h后脱水处理,常规石蜡包埋,制作3~4 μm厚度的石蜡切片,采用脱TUNEL标记凋亡的细胞核。于200倍视野计数凋亡细胞数,计算细胞凋亡率。细胞凋亡率=凋亡细胞数/总细胞数×100%。
1.5 大鼠血清中LDH和SOD活性及MDA水平大鼠腹主动脉取血,4℃条件下3 000 r·min-1离心10 min,取血清。按照试剂盒操作说明书加入相应试剂,孵育后于440 nm波长处检测各样品吸光度(A)值,根据试剂盒操作说明书提供的计算公式分别计算LDH和SOD活性及MDA水平。
1.6 大鼠心肌组织中ROS水平检测取大鼠左室心肌组织,在预冷的PBS缓冲液中漂洗,加入适量胰酶消化液,37℃消化30 min,间断性吹打已消化组织,用预冷PBS缓冲液终止消化,采用300目尼龙网过滤,收集细胞,500 r·min-1离心10 min,弃上清,重悬细胞,重悬后按照试剂盒操作说明书步骤进行,加入10 µmol·L-1 DCFH-DA荧光探针避光孵育30 min,PBS缓冲液洗涤3次,使用488 nm激发波长、525 nm发射波长,采用流式细胞仪检测,以相对平均荧光强度值表示细胞内ROS水平。
1.7 Western blotting法检测各组大鼠心肌组织中Cleaved-Caspase-3、Bax、Bcl-2、Nrf2和HO-1蛋白表达水平取大鼠左室心肌组织,称质量后加入预冷的组织蛋白裂解液和蛋白酶抑制剂匀浆,冰上碾磨组织,4℃条件下12 000×g离心20 min,收集上清,采用考马斯亮蓝法测定蛋白浓度。取20 μg蛋白进行十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)分离蛋白,并将蛋白转移至PVDF膜上,5%脱脂牛奶室温封闭1 h,分别加入Cleaved-Caspase-3、Bax、Bcl-2、Nrf2、HO-1和β-actin抗体,4℃孵育过夜。次日,加入相应二抗室温孵育1 h,ECL试剂曝光显影,在全自动凝胶成像系统中曝光显影,目的蛋白相对表达水平=目的蛋白条带灰度值/β-actin条带灰度值。
1.8 统计学分析采用SPSS 22.0统计软件进行统计学分析。各组大鼠LVSD、LVDD、EF和FS,心肌细胞凋亡率,血清中LDH和SOD活性及ROS和MDA水平,心肌组织中cleaved-Caspase-3、Bax、Bcl-2、Nrf2和HO-1蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用LSD-t检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠心功能指标与假手术组比较,模型组大鼠LVSD和LVDD明显升高(P < 0.05),EF和FS明显降低(P < 0.05);与模型组比较,高剂量QSYQ组和阳性药对照组大鼠LVSD和LVDD明显降低(P < 0.05),EF和FS明显升高(P < 0.05),低剂量QSYQ组大鼠心功能指标差异无统计学意义(P > 0.05)。见表 1。
| (n=12, x±s) | |||||||||||||||||||||||||||||
| Group | LVSD(d/mm) | LVDD(d/mm) | EF(η/%) | FS(η/%) | |||||||||||||||||||||||||
| Sham operation | 3.550±0.450 | 5.752±0.710 | 83.023±4.450 | 38.282±6.840 | |||||||||||||||||||||||||
| Model | 5.692±0.610* | 7.419±0.580* | 43.253±4.720* | 23.278±2.180* | |||||||||||||||||||||||||
| QSYQ | |||||||||||||||||||||||||||||
| Low dose | 5.127±0.680 | 7.215±0.640 | 48.727±3.180 | 28.940±4.730 | |||||||||||||||||||||||||
| High dose | 3.658±0.600△ | 6.025±0.610△ | 65.070±5.580△ | 39.286±5.680△ | |||||||||||||||||||||||||
| Positive drug control | 3.396±0.500△ | 5.724±0.710△ | 82.107±4.380△ | 40.671±4.760△ | |||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
假手术组大鼠心肌组织TUNEL染色切片中棕黄色细胞不明显,细胞凋亡率为(5.67±0.62)%;模型组大鼠心肌细胞棕黄色细胞明显增多,细胞凋亡率为(39.40±3.07)%,较假手术组明显升高(P < 0.05);高剂量QSYQ组和阳性药对照组大鼠心肌组织中棕黄色细胞明显减少,细胞凋亡率分别为(15.65±1.90)%和(9.84±0.88)%,较模型组明显降低(P < 0.05),低剂量QSYQ组大鼠肌细胞凋亡率与模型组比较差异无统计学意义(P > 0.05)。见图 1(插页三)。

|
| A: Sham operation group; B: Model group; C: Low dose of QSYQ group; D: High dose of QSYQ group; E. Positive drug group. 图 1 各组大鼠左室心肌组织细胞凋亡情况(TUNEL, ×200) Fig. 1 Apoptosis of left ventricular myocardium tissue of rats in varions groups (TUNEL, × 200) |
|
|
与假手术组比较,模型组大鼠血清中LDH活性和MDA及ROS水平明显升高(P < 0.05),SOD活性明显降低(P < 0.05);与模型组比较,高剂量QSYQ组和阳性药对照组大鼠血清中LDH活性及MDA和ROS水平明显降低(P < 0.05),SOD活性明显升高(P < 0.05),低剂量QSYQ组大鼠血清中上述各指标差异无统计学意义(P > 0.05)。见表 2。
| (n=12, x±s) | |||||||||||||||||||||||||||||
| Group | LDH[λB/(U·L-1)] | SOD[λB/(U·mL-1)] | MDA[cB/(μmol·L-1)] | ROS | |||||||||||||||||||||||||
| Sham operation | 183.310±14.640 | 168.000±5.570 | 11.133±2.920 | 1.006±0.090 | |||||||||||||||||||||||||
| Model | 504.922±38.730* | 41.667±8.150* | 94.067±2.160* | 4.749±0.250* | |||||||||||||||||||||||||
| QSYQ | |||||||||||||||||||||||||||||
| Low dose | 474.714±53.310 | 57.442±4.040 | 81.333±6.060 | 3.882±0.140 | |||||||||||||||||||||||||
| High dose | 256.028±20.840△ | 124.025±14.110△ | 33.400±3.130△ | 2.019±0.110△ | |||||||||||||||||||||||||
| Positive drug control | 224.115±13.790△ | 137.383±16.080△ | 32.367±3.480△ | 2.414±0.150△ | |||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △ P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
与假手术组比较,模型组大鼠心肌组织中Cleaved-Caspase-3和Bax蛋白表达水平明显升高(P < 0.05),Bcl-2蛋白表达水平明显降低(P < 0.05);与模型组比较,高剂量QSYQ组和阳性药卡托普利组大鼠心肌组织中Cleaved-Caspase-3和Bax蛋白表达水平明显降低(P < 0.05),Bcl-2蛋白表达水平明显升高(P < 0.05),低剂量QSYQ组大鼠心肌组织中凋亡相关蛋白表达水平差异无统计学意义(P > 0.05)。见图 2和表 3。
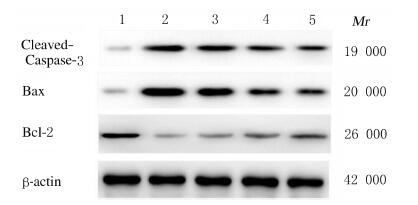
|
| Lane 1:Sham operation group; Lane 2: Model group; Lane 3: Low dose of QSYQ group; Lane 4: High dose of QSYQ group; Lane 5: Positive drug control group. 图 2 各组大鼠心肌组织中Cleaved-Caspase-3、Bax和Bcl-2蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of leaved-Caspase-3, Bax, and Bcl-2 proteins in myocardium tissue of rats in various groups |
|
|
| (n=12, x±s) | |||||||||||||||||||||||||||||
| Group | Cleaved-Caspase-3 | Bax | Bcl-2 | ||||||||||||||||||||||||||
| Sham operation | 1.044±0.050 | 0.993±0.030 | 0.997±0.040 | ||||||||||||||||||||||||||
| Model | 5.519±0.180* | 3.393±0.320* | 0.393±0.080* | ||||||||||||||||||||||||||
| QSYQ | |||||||||||||||||||||||||||||
| Low dose | 4.519±0.440 | 2.777±0.180 | 0.440±0.050 | ||||||||||||||||||||||||||
| High dose | 3.187±0.320△ | 1.908±0.260△ | 0.853±0.060△ | ||||||||||||||||||||||||||
| Positive drug control |
2.156±0.190△ | 1.357±0.180△ | 0.917±0.020△ | ||||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
与假手术组比较,模型组大鼠心肌组织中Nrf2和HO-1蛋白表达水平明显降低(P < 0.05);与模型组比较,高剂量QSYQ组大鼠心肌组织中Nrf2和HO-1蛋白表达水平明显升高(P < 0.05),阳性药对照组和低剂量QSYQ组大鼠心肌组织中Nrf2和HO-1蛋白表达水平差异无统计学意义(P > 0.05)。见图 3和表 4。
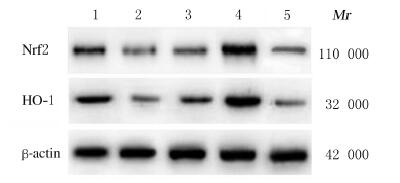
|
| Lane 1 : Sham operation group; Lane 2 : Model group; Lane 3 : Low dose of QSYQ group; Lane 4: High dose of QSYQ group; Lane 5: Positive drug control group. 图 3 各组大鼠心肌组织中Nrf2和HO-1蛋白表达电泳图 Fig. 3 Electrophoregram of expressions of Nrf2 and HO-1 proteins in myocardium tissue of rats in various groups |
|
|
| (n=12, x±s) | |||||||||||||||||||||||||||||
| Group | Nrf2 | HO-1 | |||||||||||||||||||||||||||
| Sham operation | 0.997±0.040 | 0.993±0.030 | |||||||||||||||||||||||||||
| Model | 0.260±0.040* | 0.186±0.050* | |||||||||||||||||||||||||||
| QSYQ | |||||||||||||||||||||||||||||
| Low dose | 0.415±0.020 | 0.345±0.040 | |||||||||||||||||||||||||||
| High dose | 1.732±0.140△ | 1.547±0.100△ | |||||||||||||||||||||||||||
| Positive drug control | 0.424±0.030△ | 0.217±0.060△ | |||||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with model group. | |||||||||||||||||||||||||||||
CHF是由于长期心脏负荷过重,心肌损害和收缩力减弱所致的心功能不全的一种综合征,也是众多心脏疾病的终末阶段,病死率极高[11]。我国中草药资源丰富,种类繁多,从中草药的有效成分中探寻并开发对抗心力衰竭药物意义重大。近年来有研究[12-13]报道:QSYQ在治疗心力衰竭、改善心脏功能、提高运动耐量及抑制炎症反应等方面具有良好效果。本研究采用冠状动脉前降支结扎法建立大鼠CHF模型,并给予QSYQ治疗心肌损伤,应用超声检测各组大鼠心功能,结果显示:与假手术组比较,模型组大鼠LVSD和LVDD明显升高,而EF和FS明显降低,提示CHF模型构建成功;与模型组比较,高剂量QSYQ组大鼠LVSD和LVDD明显降低,EF和FS明显升高,表明QSYQ可明显改善CHF模型大鼠心脏功能。
氧化应激是指机体在遭受各种有害刺激时,体内氧自由基产生过多,清除能力降低,导致氧化系统和抗氧化系统失衡,从而引起的氧化损伤过程。氧自由基可改变心脏结构而造成心脏功能异常,参与心力衰竭的演变进程。近年来研究[14]表明:心力衰竭动物模型中氧自由基明显增多,抵抗被氧化的能力降低。当氧自由基抵抗物质活性降低或产生过度时,将导致氧自由基沉积,进而刺激细胞膜脂质发生过氧化反应[15]。本研究结果显示:与假手术组比较,模型组大鼠血清中LDH活性及MDA和ROS水平明显升高,SOD活性降低,机体内的氧化与抗氧化平衡被打破,产生氧化应激损伤;与模型组比较,高剂量QSYQ组大鼠血清中LDH活性及MDA和ROS水平明显降低,SOD活性升高,表明QSYQ可以通过降低CHF模型大鼠机体内的氧化应激水平,缓解氧化损伤。
氧化应激可导致心肌细胞结构破坏和功能异常,进而导致心肌细胞凋亡增加,从而引起心功能损害,发生心力衰竭。细胞凋亡的发生由细胞凋亡蛋白调控,Bcl-2家族蛋白在细胞凋亡过程中起着重要作用,Bcl-2和Bax蛋白表达水平与凋亡调控直接相关。在外界刺激或Caspase-3等因子活化时,Bax从胞浆转入线粒体或细胞核膜,导致促凋亡因子与抗凋亡因子失衡,诱导细胞凋亡[16-17]。本研究采用TUNEL染色观察各组大鼠心肌组织细胞凋亡情况,结果显示:假手术组大鼠心肌组织TUNEL染色切片中棕黄色细胞不明显,细胞凋亡率低,模型组大鼠心肌组织中棕黄色细胞明显增多,细胞凋亡率明显升高,高剂量QSYQ组大鼠心肌组织中棕黄色细胞明显减少,细胞凋亡率明显降低;Western blotting实验检测结果显示:QSYQ能明显降低CHF大鼠心肌组织中促凋亡蛋白Bax和Cleaved-Caspase-3的表达水平,同时上调Bcl-2蛋白的表达水平,起到抗心肌细胞凋亡作用。
Nrf2/HO-1信号通路是迄今发现的最为重要的内源性抗氧化应激通路之一,机体内多种抗氧化酶都含有一个共同的启动子序列——抗氧化反应元件(antioxidant response element,ARE),在与ARE结合的多种转录因子中,Nrf2是目前被认为起关键作用且可被外界因素诱导的,当发生氧化应激或受到其他化学物质刺激时,Nrf2通过磷酸化与Keap1解耦连,激活Nrf2进入细胞核,与ARE特异性位点结合,进而诱导其下游表达一系列内源性的保护基因[18]。HO-1为Nrf2重要的下游靶基因,且为一种新型的心肌保护因子[19-20]。本研究结果显示:与假手术组比较,模型组大鼠心肌组织中Nrf2和HO-1蛋白表达水平明显降低;与模型组比较,阳性药对照组大鼠心肌组织中Nrf2和HO-1蛋白表达水平差异无统计学意义,可能与卡托普利的作用机制有关,其可以通过扩张血管而控制血压,减轻心脏负荷来阻滞CHF的进行性心室扩张[21]。而高剂量QSYQ组大鼠心肌组织中Nrf2和HO-1蛋白表达水平明显升高,表明QSYQ可能通过激活Nrf2/HO-1信号通路,来发挥对CHF模型大鼠心肌组织的保护作用。
综上所述,QSYQ可能通过激活Nrf2/HO-1信号通路抑制CHF模型大鼠心肌组织的氧化损伤,发挥其对心肌细胞损伤的保护作用。本研究揭示了QSYQ潜在的心肌保护作用机制,为其相关药物的开发和临床应用提供了实验依据。
| [1] |
NIE P, MENG F J, ZHANG J G, et al. Astragaloside IV exerts a myocardial protective effect against cardiac hypertrophy in rats, partially via activating the Nrf2/HO-1 signaling pathway[J]. Oxid Med Cell Longev, 2019, 2019: 4625912. |
| [2] |
马育轩, 王艳丽, 潘军英, 等. 中药治疗心血管疾病现代研究进展[J]. 中医药信息, 2018, 35(5): 109-116. |
| [3] |
李文佳, 郭迪, 王迪. 强心活力方对心肌梗死后慢性心衰大鼠心功能和氧化应激的作用研究[J]. 成都中医药大学学报, 2017, 40(3): 17-19. |
| [4] |
刘亚洋, 李鹤, 朱源生. 芪参益气滴丸对冠心病慢性心力衰竭病人心功能、免疫功能及micro RNA155水平的影响[J]. 中西医结合心脑血管病杂志, 2017, 15(11): 1342-1344. |
| [5] |
郑立文, 刘晨, 段英春, 等. 芪参益气滴丸治疗慢性心力衰竭疗效评价[J]. 吉林中医药, 2018, 38(10): 1161-1163. |
| [6] |
赵桂峰, 吴丽玉, 许传嘉. 芪参益气滴丸对心梗后大鼠心功能及心肌纤维化相关蛋白表达的影响[J]. 中国实验方剂学杂志, 2018, 24(4): 131-136. |
| [7] |
赵桂峰, 毛静远, 吴丽玉, 等. 芪参益气滴丸抑制大鼠心肌梗死后心肌纤维化及对TGF-β1/Smads通路表达的影响[J]. 中国中西医结合杂志, 2017, 37(12): 1466-1470. |
| [8] |
LU Y H, WANG D, YUAN X L, et al. Protective effect of Qishen Yiqi dropping pills on the myocardium of rats with chronic heart failure[J]. Exp Ther Med, 2019, 17(1): 378-382. |
| [9] |
OUYANG Q F, YOU T, GUO J J, et al. Effects of apelin on left ventricular-arterial coupling and mechanical efficiency in rats with ischemic heart failure[J]. Dis Markers, 2019, 2019: 4823156. |
| [10] |
尚莉莉, 全爱君, 王馨, 等. 祛痰通阳汤对慢性心力衰竭模型大鼠超声心动图及血清AngⅡ含量、心肌组织AT1 mRNA表达的影响[J]. 中医杂志, 2018, 59(1): 56-60. |
| [11] |
LEE H C, SHIOU Y L, JHUO S J, et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats[J]. Cardiovasc Diabetol, 2019, 18(1): 45-54. DOI:10.1186/s12933-019-0849-6 |
| [12] |
WANG L J, WANG L Y, ZHOU X X, et al. Qishen Yiqi Dropping Pills ameliorates doxorubicin-induced cardiotoxicity in mice via enhancement of cardiac angiogenesis[J]. Med Sci Monit, 2019, 25(3): 2435-2444. |
| [13] |
SUN J F, QIAN H, LI X G, et al. QishenYiqi Dripping Pill improves heart failure by up-regulation of β2-adrenergic receptor expression[J]. J Heart Valve Dis, 2017, 26(2): 193-199. |
| [14] |
SAHEERA S, POTNURI A G, NAIR R R. Protective effect of antioxidant Tempol on cardiac stem cells in chronic pressure overload hypertrophy[J]. Life Sci, 2019, 222: 88-93. DOI:10.1016/j.lfs.2019.02.054 |
| [15] |
CHIGNALIA A Z, ISBATAN A, PATEL M, et al. Pressure-dependent NOS activation contributes to endothelial hyperpermeability in a model of acute heart failure[J]. Biosci Rep, 2018, 38(6): BSR20181239. DOI:10.1042/BSR20181239 |
| [16] |
WANG X L, CHEN J J, WANG H B, et al. Memantine can reduce ethanol-induced caspase-3 activity and apoptosis in H4 cells by decreasing intracellular calcium[J]. J Mol Neurosci, 2017, 62(3/4): 402-411. |
| [17] |
XI H, FAN X, ZHANG Z, et al. Bax and Bcl-2 are involved in the apoptosis induced by local testicular heating in the boar testis[J]. Reprod Domest Anim, 2017, 52(3): 359-365. DOI:10.1111/rda.12904 |
| [18] |
NDISANG J F. Synergistic interaction between heme oxygenase (HO) and nuclear-factor E2-related factor-2(Nrf2) against oxidative stress in cardiovascular related diseases[J]. Curr Pharm Des, 2017, 23(10): 1465-1470. DOI:10.2174/1381612823666170113153818 |
| [19] |
SINGH N, AHMAD Z, BAID N, et al. Host heme oxygenase-1:Friend or foe in tackling pathogens[J]. IUBMB Life, 2018, 70(9): 869-880. DOI:10.1002/iub.1868 |
| [20] |
OTTERBEIN L E, FORESTI R, MOTTERLINI R. Heme oxygenase-1 and carbon monoxide in the heart:The balancing act between danger signaling and pro-survival[J]. Circ Res, 2016, 118(12): 1940-1959. DOI:10.1161/CIRCRESAHA.116.306588 |
| [21] |
AZEVEDO E R, MAK S, FLORAS J S, et al. Acute effects of angiotensin-converting enzyme inhibition versus angiotensin Ⅱ receptor blockade on cardiac sympathetic activity in patients with heart failure[J]. Am J Physiol Regul Integr Comp Physiol, 2017, 313(4): R410-R417. DOI:10.1152/ajpregu.00095.2017 |
 2020, Vol. 46
2020, Vol. 46


