扩展功能
文章信息
- 乔良伟, 曲青山, 李明
- QIAO Liangwei, QU Qingshan, LI Ming
- LncRNA-ATB通过调节miR-200c表达对肾移植大鼠急性排斥反应的影响
- Effect of LncRNA-ATB on acute rejection of kidney transplanted rats by regulating miR-200c expression
- 吉林大学学报(医学版), 2020, 46(05): 955-962
- Journal of Jilin University (Medicine Edition), 2020, 46(05): 955-962
- 10.13481/j.1671-587x.20200510
-
文章历史
- 收稿日期: 2020-02-26
肾移植是治疗终末期肾病(end-stage kidney disease,ESRD)的有效手段,排斥反应是肾移植术后的主要难题和并发症,也是影响移植肾长期存活的主要危险因素,目前临床上最常见的排斥反应类型是急性排斥反应(acute rejection,AR)[1]。近年来,随着免疫抑制剂的出现,肾移植后排斥反应发生率不断下降,但仍时有发生。长链非编码RNA(long non-coding RNA,LncRNA)是一类不具有蛋白编码功能的RNA,广泛参与调节机体的各项基础生物进程,其表达水平异常与多种疾病的发生发展存在密切关联[2]。被转化生长因子β活化的长链非编码RNA(long non-coding RNA activated by TGF-β,LncRNA-ATB)是LncRNAs的重要成员,参与细胞增殖、凋亡、转移和侵袭等多种生理过程[3]。既往研究[4]表明:LncRNA-ATB在器官移植免疫排斥反应中起调控作用,但在肾移植方面报道较少。本研究通过沉默肾移植模型大鼠LncRNA-ATB的表达,探讨LncRNA-ATB对肾移植大鼠移植后AR和受体组织炎症反应的影响,分析肾移植AR潜在的分子机制,为临床诊断和治疗AR提供参考。
1 材料与方法 1.1 实验动物、主要试剂和仪器供体:雄性SD大鼠30只,SPF级,7~8周龄,体质量250~300 g;受体:雄性Wistar大鼠40只,SPF级,7~8周龄,体质量230~300 g;均由北京维通利华实验动物技术有限公司提供,动物生产许可证号:SCXK(京)2016-0001。所有大鼠术前12 h禁食不禁水。含有LncRNA-ATB-短发夹(shRNA)和LncRNA-ATB-NC的psiCHECK2基因重组质粒(上海吉玛公司),Opti-MEM培养基和脂质体2000(Lipofectamine 2000)(美国Invitrogen公司),白细胞介素6(interleukin6,IL-6)、白细胞介素8(interleukin-8,IL-8)和肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)ELISA试剂盒(美国Abcam公司),兔抗大鼠Toll样受体4(Toll-like receptor-4,TLR4)、髓样细胞分化因子88(myeloid differentiation factor 88,MyD88)和核转录因子κB(nuclear transcription factor κB,NF-κB)多抗及辣根过氧化物酶标记的山羊抗兔MyD88和NF-κB单抗(英国Abcam公司)。7600全自动生化分析仪(日本Hitachi公司),Cary3500紫外分光光度计(美国安捷伦公司),550型全自动酶标仪和PowerPac系列电泳仪(美国Bio-Rad公司)。
1.2 大鼠肾移植AR模型建立和分组供体和受体大鼠腹腔注射水合氯醛溶液(10%W/V)进行麻醉,固定于手术台上。供体30只SD大鼠经中线切口暴露腹主动脉、左肾及其所属动静脉,结扎肾上腺动静脉,游离左肾及肾动静脉,分离输尿管,用4℃含肝素生理盐水灌注左肾阻断肾脏血液供应,切断左肾静脉,待左肾颜色变为黄白色且左肾静脉流出清亮的灌注液后,切断左肾动脉,取出肾脏保存于4℃含肝素生理盐水中,剪去供肾多余脂肪及腔静脉,以利血管吻合。受体30只Wistar大鼠暴露左肾后移除,将供肾放置左肾窝,左肾静脉采用cuff套管吻合法,动脉采用端端吻合法。吻合完成后打开血管夹,数秒内可见左肾颜色变红,少量尿液从输尿管排出。术后肌肉注射青霉素10万单位,连续3 d。其余10只Wistar大鼠为假手术组,开腹后随即连续全层关闭,其他操作同上。术后所有大鼠均给予正常饮食和饮水。术后将移植成功的27只大鼠(死亡3只)随机分为模型组、LncRNA-ATB-shRNA组和LncRNA-ATB-NC组,每组9只。
1.3 干预方法制备质粒脂质体复合物:按照Lipofectamine 2000转染试剂盒说明书,将5 μg含有LncRNA-ATB-shRNA(或LncRNA-ATB-NC)的psiCHECK2基因重组质粒、10 μL Lipofectamine 2000分别用Opti-MEM培养基稀释至总体积各为30 μL,室温静置5 min,然后将2种液体混合均匀,室温放置30 min,获得质粒脂质体复合物备用。肾移植术后2 h分别将2种质粒脂质体复合物用Opti-MEM培养基稀释至总体积200 μL,经尾静脉分别快速注入LncRNA-ATB-shRNA组和LncRNA-ATB-NC组大鼠体内。假手术组和模型组大鼠按每只200 μL尾静脉注射Opti-MEM培养基。
1.4 RT-PCR法检测大鼠外周血中LncRNA-ATB、微小RNA(microRNA,miRNA)-200c表达水平各组大鼠干预7 d后,腹主动脉采血2 mL放入抗凝管中,按照TRIzol说明书提取总RNA,应用紫外分光光度计测定RNA纯度和浓度,将提取的总RNA按试剂盒说明书进行逆转录得到相对应的cDNA,进行产物扩增。以cDNA为模板,配制反应体系:SYBR GREEN MasterMix 10 μL,上游引物0.5 μL,下游引物0.5 μL,模板cDNA 1 μL,加双蒸水至总体积20 μL。反应条件:预变性94℃、10 min;变性94℃、10 s,退火60℃、30 s,延伸70℃、20 s,35个循环,每个样本检测3次,取平均数。引物均由上海吉玛制药技术有限公司设计合成,LncRNA-ATB以β-actin为内参,miR-200c以U6为内参,采用2-△△Ct法计算LncRNA-ATB和miR-200c表达水平。引物序列见表 1。
1.5 全自动生化分析仪检测各组大鼠血肌酐(serum creatinine,Scr)和血尿素氮(blood urea nitrogen,BUN)水平各组大鼠腹主动脉采血,离心半径12 cm,3 000 r·min-1离心20 min,取上清‒20℃冰箱保存。采用全自动生化分析仪检测大鼠血清中Scr和BUN水平。
| Primer | Sequence(5'-3') |
| LncRNAATB | F:-ATTACCATCGATGCTAGCATGCA |
| R:ACAGATACCATTACATGGCGATC | |
| β-actin | F:ATTCGATCAGCTAATATCAGCGCTA |
| R:ACTGCATGCGATGCTAGCAGACTG | |
| miR-200c | F:CAGCTAGCATCGATCGCGATGAGCTA |
| R:CGCATAGCTAGCTACCATAGCAGCTA | |
| U6 | F:AACTCGATCAGATCGGCGACTACT |
| R:AGCTAGTACAGACTCGCTCATCCA |
取保存血清,严格按照ELISA试剂盒说明书操作步骤,检测大鼠血清中IL-6、IL-8和TNF-α水平,用酶标仪在490 nm波长处测量吸光度(A)值,以标准品浓度和A值绘制标准曲线,分别根据公式Y=0.970 1X+0.011 8、Y=1.025 3X-0.001 7和Y=0.974 6X+0.011 4计算待测样品IL-6、IL-8和TNF-α水平。
1.7 各组大鼠肾组织病理学观察及AR分级采血完毕,处死各组大鼠,无菌摘取左肾并切分成两半,一半置于体积分数为10%中性甲醛保存备用。取中性甲醛中保存的肾组织,常规石蜡包埋、切片、苏木精-伊红(HE)染色,置于光镜下观察大鼠肾组织病理形态表现。AR的诊断参照Banff 2007移植肾病理学分类标准[5],按照严重程度分为7级:正常、临界性改变、Ⅰa级、Ⅰb级、Ⅱa级、Ⅱb级和Ⅲ级。AR分级半定量评分:正常为0分,临界性改变为1分,Ⅰa为2分,Ⅰb级为3分,Ⅱa级为4分,Ⅱb级为5分,Ⅲ级为6分。
1.8 RT-PCR法检测各组大鼠肾组织中TLR4、MyD88中NF-κB mRNA表达水平采血完毕,处死大鼠,无菌摘取左肾并切分成两半,一半置于液氮中保存备用。取液氮中保存的肾组织100 mg,按照TRIzol试剂盒说明书提取总RNA,测定RNA纯度和浓度后进行cDNA逆转录,配制20 μL反应体系:模板cDNA 2 μL,上下游引物各2 μL,Taq DNA聚合酶10 μL,双蒸水6 μL。以转录产物cDNA为模板,以β-actin为内参,进行PCR扩增。扩增条件:预变性95℃、10 min,变性95℃、60 s,退火55℃、30 s,延伸65℃、30 s,共40个循环。各组大鼠肾组织中TLR4、MyD88和NF-κB mRNA表达水平按2-ΔΔCt法计算。引物序列见表 2。
| Gene | Sequence(5'-3') |
| TLR4 | F:5'-CTTAGATACCATCGATAATGCGCCA-3' |
| R:5'-AGCTAGCGCATAGCATCGATGACG-3' | |
| MyD88 | F:5'-ACGATTCATCGATCTGATGACTA-3' |
| R:5'-CGAACATAGGGCACTAGCTACAT-3' | |
| NF-κB | F:5'-ACGATCGAGCAGTAGCTAAATGCTATC-3' |
| R:5'-CTTACGATCGATGGATCGTAGCTAGAT-3' | |
| β-actin | F:5'-ACTTAACTAGTACTAGCGACAGG-3' |
| R:5'-CTATCAGCTATCGTCGAACTGAT-3' |
取液氮保存的肾组织100 mg,在液氮中研磨后离心取上清,BCA试剂盒检测蛋白浓度,制备蛋白样品,蛋白上样进行十二烷基硫酸钠聚丙烯酰氨(SDS-PAGE)凝胶电泳,电转至硝酸纤维素膜,5%脱脂牛奶孵育2 h,加入稀释的一抗TLR4(1︰1 000),MyD88(1︰1 000)、NF-κB(1︰1 000)和β-actin(1︰1 000),4℃摇床封闭过夜,洗膜后加入稀释的二抗(1︰4 000)于室温下摇床孵育1 h,洗膜后加入ELC试剂显影,暗室下曝光成像。采用Image J软件分析图像,计算TLR4、MyD88和NF-κB蛋白表达水平。目的蛋白表达水平=目的蛋白条带灰度值/β-actin蛋白条带灰度值。
1.10 统计学分析采用SPSS 21.0统计软件进行统计学分析。采用KS检验数据正态分布,各组大鼠外周血中LncRNA-ATB和miR-200c表达水平,血清中Scr、BUN,IL-6、IL-8和TNF-α水平,肾组织中TLR4、MyD88和NF-κB mRNA及蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析,两两组间样本均数比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组大鼠外周血中LncRNA-ATB和miR-200c表达水平各组大鼠外周血中LncRNA-ATB和miR-200c表达水平组间比较差异均有统计学意义(P < 0.01)。与假手术组比较,模型组、LncRNA-ATB-NC组和LncRNA-ATB-shRNA组大鼠外周血中LncRNA-ATB表达水平均升高(P < 0.05),miR-200c表达水平均降低(P < 0.05);与模型组和LncRNA-ATB-NC组比较,LncRNA-ATB-shRNA组LncRNA-ATB表达水平降低(P < 0.05),miR-200c表达水平升高(P < 0.05);模型组与LncRNA-ATB-NC组大鼠外周血中LncRNA-ATB和miR-200c表达水平比较差异无统计学意义(P > 0.05)。见表 3。
| (x±s) | |||||||||||||||||||||||||||||
| Group | n | LncRNA-ATB | miR-200c | ||||||||||||||||||||||||||
| Sham operation | 10 | 0.78±0.08 | 0.94±0.11 | ||||||||||||||||||||||||||
| Model | 9 | 2.31±0.25* | 0.32±0.04* | ||||||||||||||||||||||||||
| LncRNA-ATB-NC | 9 | 2.26±0.23* | 0.29±0.03* | ||||||||||||||||||||||||||
| LncRNA-ATB-shRNA | 9 | 1.32±0.15*△# | 0.57±0.06*△# | ||||||||||||||||||||||||||
| F | 149.884 | 181.786 | |||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | |||||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with model group; #P < 0.05 compared with LncRNA-ATB-NC group. | |||||||||||||||||||||||||||||
各组大鼠血清中Scr、BUN、IL-6、IL-8和TNF-α水平组间比较差异有统计学意义(P < 0.01)。与假手术组比较,模型组、LncRNA-ATB-NC组和LncRNA-ATB-shRNA组大鼠血清中Scr、BUN、IL-6、IL-8和TNF-α水平均升高(P < 0.05);与模型组和LncRNA-ATB-NC组比较,LncRNA-ATB-shRNA组大鼠血清中Scr、BUN、IL-6、IL-8和TNF-α水平均降低(P < 0.05);模型组与LncRNA-ATB-NC组大鼠血清中Scr、BUN、IL-6、IL-8和TNF-α水平比较差异无统计学意义(P > 0.05)。见表 4。
| (x±s) | |||||||||||||||||||||||||||||
| Group | n | Scr [cB/(μmol·L-1)] |
BUN [cB/(mmol·L-1)] | IL-6 [ρB/ (mg·L-1)] |
IL-8 [ρB/ (mg·L-1)] |
TNF-α [ρB/ (mg·L-1)] | |||||||||||||||||||||||
| Sham operation | 10 | 73.64±9.51 | 8.56±1.39 | 65.26±7.02 | 215.61±22.75 | 45.31±4.75 | |||||||||||||||||||||||
| Model | 9 | 152.43±16.01* | 49.52±5.51* | 133.43±12.56* | 268.52±27.51* | 128.52±13.21* | |||||||||||||||||||||||
| LncRNA-ATB-NC | 9 | 148.82±15.73* | 53.21±6.02* | 125.68±12.73* | 273.49±28.46* | 131.27±14.08* | |||||||||||||||||||||||
| LncRNA-ATB-shRNA | 9 | 109.68±10.22*△# | 20.15±2.87*△# | 89.65±10.27*△# | 238.47±24.87*△# | 78.52±8.47*△# | |||||||||||||||||||||||
| F | 76.291 | 242.250 | 82.377 | 10.416 | 143.621 | ||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with model group; #P < 0.05 compared with LncRNA-ATB-NC group. | |||||||||||||||||||||||||||||
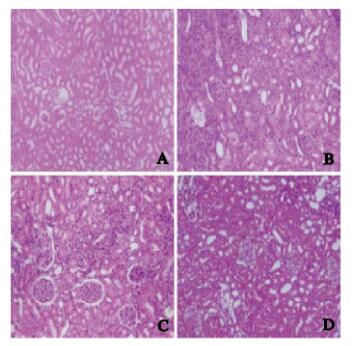
HE染色,假手术组大鼠肾组织无AR,结构清楚;模型组大鼠出现典型AR,表现为炎性细胞浸润、动脉炎症、间质细胞水肿和皮质出血等;LncRNA-ATB-NC组与模型组肾组织病理形态表现相似;LncRNA-ATB-shRNA组大鼠肾组织病理形态表现较模型组和LncRNA-ATB-NC组均减轻。假手术组、模型组、LncRNA-ATB-NC组和LncRNA-ATB-shRNA组的AR分级半定量评分分别为(0.00±0.00)分、(4.26±0.44)分、(4.54±0.45)分和(1.75±0.18)分。AR分级半定量评分组间比较差异均有统计学意义(F=425.923,P < 0.05)。与假手术组比较,模型组、LncRNA-ATB-NC组和LncRNA-ATB-shRNA组的AR分级半定量评分均升高(P < 0.05);与模型组和LncRNA-ATB-NC组比较,LncRNA-ATB-shRNA组的AR分级半定量评分降低(P < 0.05);模型组与LncRNA-ATB-NC组的AR分级半定量评分比较差异无统计学意义(P > 0.05)。见图 1(插页二)。
|
| A: Sham operation group; B: Model group; C: LncRNA-ATB NC group; D: LncRNA-ATB-shRNA group. 图 1 各组大鼠肾组织病理形态表现(HE, × 200) Fig. 1 Pathomorphology of kidney tissue of rats in various groups (HE, × 200) |
|
|
各组大鼠肾组织中TLR4、MyD88和NF-κB mRNA相对表达水平组间比较差异有统计学意义(P < 0.01)。与假手术组比较,模型组、LncRNA-ATB-NC组和LncRNA-ATB-shRNA组大鼠肾组织中TLR4、MyD88和NF-κB mRNA表达水平均升高(P < 0.05);与模型组和LncRNA-ATB-NC组比较,LncRNA-ATB-shRNA组大鼠肾组织中TLR4、MyD88和NF-κB mRNA表达水平均降低(P < 0.05);模型组与LncRNA-ATB-NC组大鼠肾组织中TLR4、MyD88和NF-κB mRNA表达水平比较差异无统计学意义(P > 0.05)。见表 5。
| (x±s) | |||||||||||||||||||||||||||||
| Group | n | TLR4 | MyD88 | NF-κB | |||||||||||||||||||||||||
| Sham operation | 10 | 0.68±0.07 | 0.64±0.08 | 0.73±0.09 | |||||||||||||||||||||||||
| Model | 9 | 1.07±0.12* | 1.13±0.11* | 1.25±0.13* | |||||||||||||||||||||||||
| LncRNA-ATB-NC | 9 | 1.12±0.13* | 1.08±0.12* | 1.16±0.14* | |||||||||||||||||||||||||
| LncRNA-ATB-shRNA | 9 | 0.89±0.09*△# | 0.87±0.09*△# | 0.95±0.11*△# | |||||||||||||||||||||||||
| F | 34.913 | 47.159 | 36.608 | ||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with model group; #P < 0.05 compared with LncRNA-ATB-NC group. | |||||||||||||||||||||||||||||
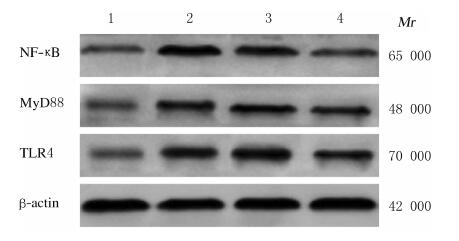
各组大鼠肾组织中TLR4、MyD88和NF-κB蛋白表达水平组间比较差异均有统计学意义(P < 0.01)。与假手术组比较,模型组、LncRNA-ATB-NC组和LncRNA-ATB-shRNA组大鼠肾组织中TLR4、MyD88和NF-κB蛋白表达水平均升高(P < 0.05);与模型组和LncRNA-ATB-NC组比较,LncRNA-ATB-shRNA组大鼠肾组织中TLR4、MyD88和NF-κB蛋白表达水平均降低(P < 0.05);模型组与LncRNA-ATB-NC组大鼠肾组织中TLR4、MyD88和NF-κB蛋白表达水平比较差异无统计学意义(P > 0.05)。见表 6和图 2。
| (x±s) | |||||||||||||||||||||||||||||
| Group | n | TLR4 | MyD88 | NF-κB | |||||||||||||||||||||||||
| Sham operation | 10 | 0.35±0.04 | 0.32±0.03 | 0.24±0.03 | |||||||||||||||||||||||||
| Model | 9 | 0.81±0.08* | 0.78±0.09* | 0.85±0.09* | |||||||||||||||||||||||||
| LncRNA-ATB-NC | 9 | 0.85±0.09* | 0.76±0.08* | 0.79±0.08* | |||||||||||||||||||||||||
| LncRNA-ATB-shRNA | 9 | 0.59±0.07*△# | 0.57±0.06*△# | 0.43±0.05*△# | |||||||||||||||||||||||||
| F | 98.609 | 94.539 | 183.702 | ||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||||
| *P < 0.05 compared with sham operation group; △P < 0.05 compared with model group; #P < 0.05 compared with LncRNA-ATB-NC group. | |||||||||||||||||||||||||||||
|
| Lane 1 : Sham operation group; Lane 2 : Model group; Lane 3 : LncRNA-ATB-NC group; Lane 4: LncRNA-ATB-shRNA group. 图 2 各组大鼠肾组织中NF-κB、MyD88和TLR4蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of NF-κB, MyD88, and TLR4 proteins in kidney tissue of rats in various groups |
|
|
ERSD是全球范围内重大公共卫生问题,随着肾移植的逐渐推广,部分患者得到了有效治疗,但术后AR仍然是一个不可忽视的问题,其不仅是造成移植肾失功的危险因素,同时也严重影响移植肾的长期存活。随着免疫抑制剂的不断出现和改进,器官移植排斥反应发生率明显下降,但大量使用免疫抑制剂会给患者带来不良反应[6]。因此,探讨移植免疫相关的分子机制对肾移植AR早期诊断和治疗具有重要意义。LncRNA和miRNA是最重要的2类非编码RNA,均在基因表达调控中起着关键作用。多项研究[7-9]表明:器官移植后LncRNA在免疫排斥受体体内异常表达,可以利用LncRNA作为早期诊断受体对同种异体移植物产生AR的标志物。既往研究[10-12]表明:LncRNA可作为内源竞争性RNA(competing endogenous RNA,ceRNA)与miRNA直接结合,从而抑制miRNA的活性,调控下游信号通路,进而影响疾病发展。研究[13]显示:LncRNA-ATB与miR-200s也存在上述相互作用,LncRNA-ATB可以与miR-200s结合,抑制miR-200s的表达,促进细胞增殖、迁移和侵袭。
本研究采用同种异体大鼠建立肾移植AR模型,与假手术组比较,模型组大鼠外周血LncRNA-ATB表达水平明显升高,表现出AR典型病理变化,经shRNA干扰载体沉默LncRNA-ATB后,LncRNA-ATB-shRNA组大鼠外周血中LncRNA-ATB表达水平、AR半定量评分及血清中Scr和BUN水平明显降低,肾组织AR病理形态改变减轻,提示肾移植AR大鼠LncRNA-ATB异常升高,可能参与AR发生发展过程,沉默LncRNA-ATB后可减弱肾移植后AR的发展。研究[14]表明:在肾移植术后AR受体肾组织切片中LncRNA-ATB表达水平明显高于对照组,促进供体器官炎症反应。QIU等[15]在对肾移植AR患者和对照肾移植患者的肾脏活检组织中检测到LncRNA-ATB表达,AR患者LncRNA-ATB表达水平较对照组明显上调,与本研究结果类似,进一步提示LncRNA-ATB可能参与大鼠肾移植术后AR进展,沉默LncRNA-ATB有利于减弱AR发展,减轻肾功能损害。
本研究结果显示:与模型组比较,LncRNA-ATB-shRNA组大鼠外周血中miR-200c表达水平明显升高,提示LncRNA-ATB可能参与调控miR-200c在肾移植大鼠中的表达。刘易[16]研究发现:LncRNA-ATB参与调控miR-200c的表达,LncRNA-ATB可以作为ceRNA竞争性结合miR-200c促进肺上皮细胞纤维化。赵伟等[17]研究显示:LncRNA-ATB与miR-200c竞争性地调控其下游通路发挥对miR-200c的调控作用。本研究与上述研究结果一致,表明LncRNA-ATB与miR-200c在疾病发生发展过程中有关联。miR-200c是一种能够抑制炎症反应的miRNA,与TLR4信号通路关系密切。TLR4信号通路与炎症反应密切相关,在依赖MyD88途径中,通过活化的结构域与MyD88结合,释放NF-κB,活化下游炎症因子(IL-6、IL-8和TNF-α等),促进炎症反应。过表达miR-200c可抑制TLR4信号通路,下调MyD88表达,阻断NF-κB活化,降低IL-6和TNF-α表达[18]。既往研究[19]显示:miR-200c可以通过改变NF-κB活性来调节IL-8表达,过表达miR-200c可以降低IL-8表达。在口腔鳞状细胞癌中,NF-κB激活的同时伴有促炎miRNA表达水平升高及miR-200c表达抑制[20]。本研究中经shRNA干扰载体沉默LncRNA-ATB后,大鼠外周血中miR-200c表达水平明显升高,IL-6、IL-8和TNF-α水平,TLR4、MyD88和NF-κB mRNA及蛋白表达水平均降低,提示沉默LncRNA-ATB可能通过上调miR-200c表达抑制TLR4/MyD88信号通路发挥抗炎作用。
综上所述,在大鼠肾移植后,沉默LncRNA-ATB可发挥抑制AR及炎症反应发生作用,其机制可能是通过上调miR-200c表达,阻断下游TLR4/MyD88信号通路,进而减弱AR发展,抑制受体组织炎症反应,本研究结果为临床早期诊断和治疗肾移植术后AR提供一定理论依据。
| [1] |
中华医学会器官移植学分会, 中国医师协会器官移植医师分会. 中国肾移植排斥反应临床诊疗指南(2016版)[J]. 器官移植, 2016, 7(5): 332-338. |
| [2] |
RAMNARINE V R, KOBELEV M, GIBB E A, et al. The evolution of long noncoding RNA acceptance in prostate cancer initiation, progression, and its clinical utility in disease management[J]. Eur Urol, 2019, 76(5): 546-559. DOI:10.1016/j.eururo.2019.07.040 |
| [3] |
JANG S Y, KIM G, PARK S Y, et al. Clinical significance of LncRNA-ATB expression in human hepatocellular carcinoma[J]. Oncotarget, 2017, 8(45): 78588-78597. DOI:10.18632/oncotarget.21094 |
| [4] |
CHEN W B, PENG W J, HUANG J R, et al. Microarray analysis of long non-coding RNA expression in human acute rejection biopsy samples following renal transplantation[J]. Mol Med Rep, 2014, 10(4): 2210-2216. DOI:10.3892/mmr.2014.2420 |
| [5] |
SOLEZ K, COLVIN R B, RACUSEN L C, et al. Banff 07 classification of renal allograft pathology:updates and future directions[J]. Am J Transplant, 2008, 8(4): 753-760. DOI:10.1111/j.1600-6143.2008.02159.x |
| [6] |
王琴, 杨春兰, 冯丽娟, 等. 基因多态性与器官移植受者霉酚酸个体化治疗研究进展[J]. 安徽医科大学学报, 2018, 53(1): 161-166. |
| [7] |
ZOU Y, ZHANG W, ZHOU H H, et al. Analysis of long noncoding RNAs for acute rejection and graft outcome in kidney transplant biopsies[J]. Biomark Med, 2019, 13(3): 185-195. DOI:10.2217/bmm-2018-0272 |
| [8] |
GU G X, HUANG Y J, WU C L, et al. Differential expression of long noncoding RNAs during cardiac allograft rejection[J]. Transplantation, 2017, 101(1): 83-91. DOI:10.1097/TP.0000000000001463 |
| [9] |
GE Y Z, XU T, Cao W J, et al. A molecular signature of two long non-coding RNAs in peripheral blood predicts acute renal allograft rejection[J]. Cell Physiol Biochem, 2017, 44(3): 1213-1223. DOI:10.1159/000485451 |
| [10] |
张扬.沉默LncRNA-ATB靶向miR-141-3p抑制乳腺癌的转移和侵袭[D].沈阳: 中国医科大学, 2018.
|
| [11] |
SONG Y X, YANG L X, GUO R W, et al. Long noncoding RNA MALAT1 promotes high glucose-induced human endothelial cells pyroptosis by affecting NLRP3 expression through competitively binding miR-22[J]. Biochem Biophys Res Commun, 2019, 509(2): 359-366. DOI:10.1016/j.bbrc.2018.12.139 |
| [12] |
TANG F C, LU Z C, WANG J M, et al. Competitive endogenous RNA(ceRNA) regulation network of LncRNAs, miRNAs, and mRNAs in Wilms tumour[J]. BMC Med Genomics, 2019, 12(1): 194. DOI:10.1186/s12920-019-0644-y |
| [13] |
HAN F, WANG C H, WANG Y, et al. Long noncoding RNA ATB promotes osteosarcomacell proliferation, migration and invasion by suppressing miR-200s[J]. Am J Cancer Res, 2017, 7(4): 770-783. |
| [14] |
LORENZEN J M, SCHAUERTE C, KÖLLING M, et al. Long Noncoding RNAs in urine are detectable and may enable early detection of acute T cell-mediated rejection of renal allografts[J]. Clin Chem, 2015, 61(12): 1505-1514. DOI:10.1373/clinchem.2015.243600 |
| [15] |
QIU J, CHEN Y, HUANG G, et al. Transforming growth factor-β activated long non-coding RNA ATB plays an important role in acute rejection of renal allografts and may impacts the postoperative pharmaceutical immunosuppression therapy[J]. Nephrology (Carlton), 2017, 22(10): 796-803. DOI:10.1111/nep.12851 |
| [16] |
刘易. LncRNA-ATB竞争性吸附miR-200c调控EMT促进矽尘诱导肺纤维化的机制研究[D].南京: 南京医科大学, 2018.
|
| [17] |
赵伟, 李晓明. 被转化生长因子β活化的长链非编码RNA对人淋巴瘤Raji细胞增殖、周期及凋亡的影响[J]. 中国现代医学杂志, 2017, 9(5): 91-96. |
| [18] |
WENDLANDT E B, GRAFF J W, GIOANNINI T L, et al. The role of microRNAs miR-200b and miR-200c in TLR4 signaling and NF-κB activation[J]. Innate Immun, 2012, 18(6): 846-855. DOI:10.1177/1753425912443903 |
| [19] |
CHUANG T D, HO M, KHORRAM O. The regulatory function of miR-200c on inflammatory and cell-cycle associated genes in SK-LMS-1, a leiomyosarcoma cell line[J]. Reprod Sci, 2015, 22(5): 563-571. DOI:10.1177/1933719114553450 |
| [20] |
JOHNSON J J, MILLER D L, JIANG R, et al. Protease-activated receptor-2(PAR-2)-mediated Nf-κB activation suppresses inflammation-associated tumor suppressor MicroRNAs in oral squamous cell carcinoma[J]. J Biol Chem, 2016, 291(13): 6936-6945. DOI:10.1074/jbc.M115.692640 |
 2020, Vol. 46
2020, Vol. 46


