扩展功能
文章信息
- 王明月, 王浩, 王冬梅, 杨泽斌, 崔梅英, 刘宁, 黄莉莉, 关新刚
- WANG Mingyue, WANG Hao, WANG Dongmei, YANG Zebin, CUI Meiying, LIU Ning, HUANG Lili, GUAN Xingang
- SIRPα-GFP真核表达载体构建及其在HEK293T细胞中的表达
- Construction of SIRPα-GFP eukaryotic expression vector and its expression in HEK293T cells
- 吉林大学学报(医学版), 2020, 46(05): 925-929
- Journal of Jilin University (Medicine Edition), 2020, 46(05): 925-929
- 10.13481/j.1671-587x.20200505
-
文章历史
- 收稿日期: 2020-02-02
信号调节蛋白α(signal regulatory protein-α,SIRPα),也被称为CD172a或SHPS-1,是属于免疫球蛋白超家族中的一种膜蛋白,是一种在巨噬细胞、树突状细胞和中性粒细胞等髓系细胞上表达的抑制性受体分子[1]。CD47是SIRPα的天然配体,在多种生理病理过程中均发挥作用[2-4]。正常的血液系统细胞表面均有CD47表达,CD47-SIRPα通路在红细胞老化吞噬过程中发挥作用[5]。SIRPα作为巨噬细胞上的关键免疫抑制受体,与SIRPα配体CD47的相互作用可阻止自体吞噬作用,因此可以利用CD47与SIRPα识别抑制巨噬细胞的吞噬作用和移植排斥反应[6-7]。SIRPα除在巨噬细胞表面表达外,还在中性粒细胞的表面呈高水平表达,多种肿瘤中CD47分子呈高水平表达,巨噬细胞和中性粒细胞表面的SIRPα与其识别结合后,会介导免疫抑制,发生免疫逃逸,从而促进肿瘤的发展[8-9]。CD47-SIRPα信号通路为肿瘤治疗提供了新的思路,通过阻断CD47-SIRPα信号通路激活吞噬细胞对肿瘤的吞噬,增强肿瘤抗原递呈,引发机体抗肿瘤免疫反应,杀伤肿瘤细胞[10-11]。本研究通过构建融合表达绿色荧光蛋白(green fluorescent protein,GFP)的SIRPα真核表达载体,制备稳定表达SIRPα-GFP的HEK293T细胞系,并研究SIRPα-GFP在细胞中的表达定位,为后续SIRPα的功能研究以及CD47-SIRPα信号通路阻断研究奠定基础。
1 材料与方法 1.1 细胞、主要试剂和仪器HEK293T细胞为本实验室冻存。培养基购自美国Hyclone公司,胎牛血清购自杭州四季青公司,pLenti-C-mGFP质粒和pCMV6-SIRPα质粒购自美国Origene公司,MluⅠ和SgfⅠ限制性内切酶购自日本TaKaRa公司,T4 DNA连接酶购自上海碧云天生物技术公司,DH5α感受态细胞和卡那霉素购自生工生物工程(上海)股份有限公司,质粒提取试剂盒和凝胶回收试剂盒购自美国Axyprep公司,Lipofectamine 3000转染试剂购自美国Invitrogen公司,单克隆SIRPα抗体购自美国Biolegend公司,蛋白预染Marker、辣根过氧化物酶标记山羊抗小鼠IgG(H+L)和显色底物购自上海碧云天生物技术有限公司。核酸电泳系统、凝胶成像系统、蛋白电泳和转膜系统均购自美国Bio-Rad公司,倒置荧光显微镜购自美国Lifte-Technologies公司,全自动多功能酶标仪购自瑞士TECAN公司。
1.2 SIRPα真核表达载体的构建和鉴定将测序正确的pLenti-C-mGFP质粒与pCMV6-SIRPα质粒用限制性内切酶MluⅠ和SgfⅠ进行双酶切:各取2 μg质粒,加入1 μL限制性内切酶MluⅠ和SgfⅠ,37℃水浴4 h,酶切产物经0.8%琼脂糖凝胶电泳分离并对相关片段进行凝胶回收,利用T4 DNA快速连接酶在25℃水浴连接1 h,将连接产物转化到DH5α感受态细胞,接种到含有卡那霉素的LB平板培养基中,过夜培养。从平板培养基中挑取单个菌落,160 r·min摇床过夜,提取质粒后用MluⅠ和SgfⅠ限制性内切酶进行双酶切,在0.8%琼脂糖凝胶电泳检测双酶切后DNA片段大小。双酶切验证成功的质粒DNA送到生工生物工程(上海)股份有限公司进行DNA测序分析。
1.3 重组质粒转染HEK293T细胞HEK293T细胞在含有10%胎牛血清的DMEM培养基中,置于37℃、5% CO2的培养箱中培养。转染前1 d,将对数生长期的HEK293T细胞以每孔1×106个细胞接种于6孔细胞培养板中,使细胞在第2天达到70%~80%融合度。第2天,从培养箱中取出6孔细胞培养板培养的HEK293T细胞,吸除原有培养基,加入2 mL PBS缓冲液清洗细胞2次后,换为Opti-MEM培养基培养。利用Lipofectamine 3000转染试剂,将2 μg质粒DNA以及10 μL转染试剂分别与125 μL Opti-MEM培养基混匀,之后将带有质粒DNA的混合物加入到带有转染试剂的试管中,混匀后室温静置15 min,加入到6孔细胞培养板HEK293T细胞培养基中。转染48 h后在荧光显微镜下488 nm激发光观察SIRPα-GFP绿色荧光的表达及细胞膜定位情况。
1.4 Western blotting法检测HEK293T细胞中SIRPα-GFP融合蛋白的表达为了对转染后的HEK293T细胞进行筛选,转染48 h后将细胞转移到10 cm培养皿进行培养,使细胞在24 h后达到40%~50%的融合度,更换为含有嘌呤霉素(8 mg·L-1)的新鲜培养基,每隔2 d弃去原有培养基,用PBS缓冲液清洗2遍,更换含有嘌呤霉素(8 mg·L)的新鲜培养基,此步骤重复3次。已经筛选后的细胞,扩大培养后收集细胞,PBS缓冲液清洗2~3次,用RIPA裂解液冰上裂解60 min,每隔10 min颠倒混匀,之后将裂解物经12 000 g、4℃离心30 min,留取上清进行蛋白定量以及Western blotting法验证。取30 μg总蛋白经12% SDS-PAGE凝胶分离,25 V恒压14 min转移到PVDF膜上。PVDF膜用含有5%脱脂奶粉的TBST封闭液室温封闭2 h,与稀释后的SIRPα一抗(1:1000稀释)和Actin一抗(1:1 000稀释)在4℃摇床孵育过夜。过夜后的PVDF膜用TBST清洗3次,每次10 min,再与羊抗鼠二抗(1:1 000稀释)室温孵育2 h,经TBST洗膜3次,每次10 min。将膜放置于显影仪曝光板上,将特超敏ECL化学发光试剂盒(BeyoECL Star)中的A液和B液按1:1比例混合,覆盖到膜上,曝光30 s,拍摄图像,若在相对分子质量为83 000附近出现清晰条带则提示SIRPα-GFP融合蛋白表达成功。
2 结果 2.1 SIRPα-GFP真核表达载体的构建本研究利用现有的慢病毒质粒pLenti-C-mGFP作为真核表达载体进行重组质粒构建。将表达载体pLenti-C-mGFP与SIRPα质粒(pCMV6-SIRPα)用限制性内切酶MluⅠ和SgfⅠ双酶切后,酶切产物经0.8%琼脂糖凝胶电泳分离,pLenti-C-mGFP质粒双酶切后可见长度约为756 bp的片段,pCMV6-SIRPα质粒双酶切后可见长度约为1 527 bp的片段(图 1)。目的片段凝胶回收后,连接产物转化Kana平板,阳性菌落在进行质粒提取后通过MluⅠ和SgfⅠ双酶切鉴定,电泳结果显示:重组质粒双酶切后可见约为7 800和1 500 bp的2个DNA条带,与pLenti-C-mGFP载体及SIRPα基因大小相符(图 2),提示重组质粒pLenti-SIRPα-GFP构建成功。
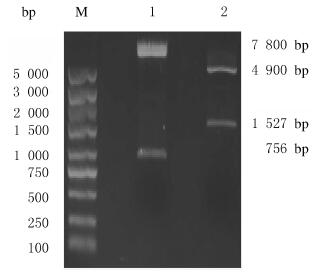
|
| M:DL 5 000 DNA marker; Lane 1:pLenti-C-mGFP vector; Lane 2:pCMV6-SIRPα plasmid. 图 1 GFP表达载体(pLenti-C-mGFP)和SIRPα质粒(pCMV6-SIRPα)双酶切结果 Fig. 1 Double digestion results of GFP expression vector(pLenti-C-mGFP) and SIRPα plasmid(pCMV6-SIRPα) |
|
|
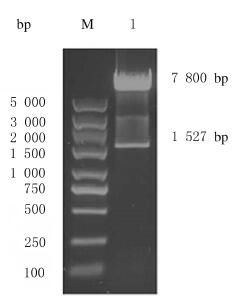
|
| M:DL 5 000 DNA marker; Lane 1:pLenti-SIRPα-GFP. 图 2 重组质粒pLenti-SIRPα-GFP的双酶切鉴定 Fig. 2 Identification of recombinant plasmid pLenti-SIRPα-GFP by double digestion |
|
|
将重组质粒pLenti-SIRPα-GFP进行DNA序列测定分析,测序结果显示:SIRPα基因成功插入到pLenti-C-mGFP载体中的SgfⅠ位点(GCGATCGCC),提示SIRPα-GFP重组质粒pLenti-SIRPα-GFP构建成功(图 3,见插页一)。
|
| M:DL 5 000 DNA marker; Lane 1:pLenti-SIRPα-GFP. 图 3 重组质粒pLenti- SIRPa GFP的测序结果 Fig. 3 Sequencing results of recombinant plasmid pLenti- SIRPa-GFP(seen on page 927 in paragraph) |
|
|
通过Lipofectamine 3000转染试剂将重组质粒pLenti-SIRPα-GFP转染HEK293T细胞,48 h后在荧光显微镜下观察绿色荧光。绿色荧光清晰地表达在转染后的HEK293T细胞膜上,与文献[9]报道的SIRPα蛋白的膜定位一致。见图 4(插页一)。
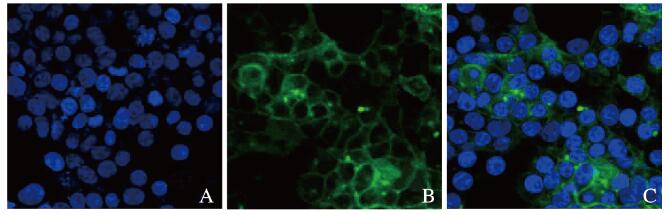
|
| A:DAPI; B:SIRPa-GFP; C:Merge. 图 4 荧光显微镜观察SIRPa-GFP在稳定转染HEK293T细胞中的表达(Bar= 20 μm) Fig. 4 Expression of SIRPa-GFP in HEK293T cells stably transfected with pLenti- SIRPa GFP observed by fluorescence microscope(Bar= 20 μm)(seen on page 927 in paragraph) |
|
|
转染空载体pLenti-C-mGFP的HEK293T细胞及转染pLenti-SIRPα-GFP后的HEK293T细胞裂解后,采用Western blotting法检测HEK293T细胞中SIRPα蛋白的表达,结果显示:转染pLenti-SIRPα-GFP质粒的HEK293T细胞裂解液在相对分子质量为83 000附近出现清晰的条带,转染空质粒的HEK293T细胞裂解液则未检测出,考虑到SIRPα和GFP蛋白的相对分子质量分别为56 000及27 000,提示利用SIRPα抗体检测的特异性条带为SIRPα-GFP融合蛋白。Western blotting法检测结果表明:SIRPα-GFP融合蛋白在HEK293T细胞中成功表达。见图 5。

|
| Lane 1:pLenti-C-mGFP transfected cells; Lane 2:pLenti-SIRPα-GFP transfected cells. 图 5 Western blotting法检测HEK293T细胞中SIRPα-GFP蛋白表达电泳图 Fig. 5 Electrophoregram of expression of SIRPα-GFP protein in HEK293T cells detected by Western blotting method |
|
|
肿瘤细胞通过表达与免疫细胞表面受体相互作用的活化或抑制性配体来逃避免疫监视[12]。肿瘤与免疫细胞之间的相互作用可防止肿瘤被免疫系统杀伤,促进肿瘤细胞的增殖[13-14]。肿瘤细胞通过过度表达的CD47分子识别免疫细胞表面表达的SIRPα分子,使肿瘤能够逃避免疫系统监视,因此CD47-SIRPα信号通路在肿瘤治疗等方面作用的研究受到越来越多的关注[15-16]。SIRPα是SIRP家族中一个典型的抑制性受体,在巨噬细胞、树突状细胞、嗜中性粒细胞和神经元上具有高水平的表达,研究[17]表明:SIRPα在生理和病理过程中均发挥重要作用。SIRPα代表一种髓样特异性免疫检查点,与肿瘤细胞表面高表达的“不要吃我”信号CD47结合,会抑制免疫系统对肿瘤细胞的攻击[18]。因此阻断SIRPα与CD47的识别,会切断CD47-SIRPα信号通路对免疫系统的抑制,恢复免疫细胞的吞噬杀伤作用,达到治疗肿瘤的目的[19-20]。
GFP作为示踪标记,对宿主细胞不具有毒性,不会影响目的蛋白的表达,可以直接在显微镜下观察融合蛋白表达和定位,因此在融合蛋白真核表达研究中具有不可替代的作用[21-23]。由于HEK293细胞生长繁殖速度快且极少表达细胞外配体所需的内生受体,且比较容易转染[24-25],因此选用HEK293T细胞作为目的细胞。本研究构建SIRPα-GFP真核表达载体,利用GFP起到示踪的目的,荧光显微镜下观察HEK293T细胞中融合蛋白的表达及定位,结果表明:SIRPα-GFP融合蛋白在HEK293T细胞膜上表达;Western blotting法检测结果进一步验证了重组质粒SIRPα-GFP转染HEK293T细胞后融合蛋白的表达。
综上所述,本研究成功构建了SIRPα-GFP真核表达载体,并利用脂质体转染试剂成功转染HEK293T细胞,SIRPα-GFP融合蛋白在HEK293T细胞中表达并位于HEK293T细胞膜上,为后续SIRPα的功能研究奠定基础。
| [1] |
ZHAO X W, VAN BEEK E M, SCHORNAGEL K, et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction[J]. Proc Natl Acad Sci U S A, 2011, 108(45): 18342-18347. DOI:10.1073/pnas.1106550108 |
| [2] |
ANDRECHAK J C, DOOLING L J, DISCHER D E. The macrophage checkpoint CD47: SIRPα for recognition of 'self' cells: from clinical trials of blocking antibodies to mechanobiological fundamentals[J]. Philos Trans R Soc Lond BBiol Sci, 2019, 374(1779): 20180217. DOI:10.1098/rstb.2018.0217 |
| [3] |
OLDENBORG P A, GRESHAM H D, LINDBERG F P. CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis[J]. J Exp Med, 2001, 193(7): 855-862. DOI:10.1084/jem.193.7.855 |
| [4] |
张帆, 何萌, 李元, 等. miR-17-5p靶向信号调节基因SIRPα调控巨噬细胞抗结核分枝杆菌的炎症反应[J]. 生物技术, 2020, 30(1): 30-37. |
| [5] |
ULUTZ H. Comment concerning the tole of CD47 and signal regulatory protein alpha in regulating the clearance of aged red blood cells[J]. Transfus Med Hemother, 2013, 40(2): 140-141. DOI:10.1159/000350507 |
| [6] |
IDE K, WANG H, TAHARA H, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages[J]. Proc Natl Acad Sci U S A, 2007, 104(12): 5062-5066. DOI:10.1073/pnas.0609661104 |
| [7] |
LI D Y, XIE S L, WANG G Y, et al. CD47 blockade alleviates acute rejection of allogeneic mouse liver transplantation by reducing ischemia/reperfusion injury[J]. Biomed Pharmacother, 2020, 123: 109793. DOI:10.1016/j.biopha.2019.109793 |
| [8] |
YANG H C, SHAO R Y, HUANG H X, et al. Engineering macrophages to phagocytose cancer cells by blocking the CD47/SIRPɑ axis[J]. Cancer Med, 2019, 8(9): 4245-4253. DOI:10.1002/cam4.2332 |
| [9] |
KOH E, LEE E J, NAM G H, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis[J]. Biomaterials, 2017, 121: 121-129. DOI:10.1016/j.biomaterials.2017.01.004 |
| [10] |
HUANG Y, LV S Q, LIU P Y, et al. A SIRPα-Fc fusion protein enhances the antitumor effect of oncolytic adenovirus against ovarian cancer[J]. Mol Oncol, 2020, 14(3): 657-668. DOI:10.1002/1878-0261.12628 |
| [11] |
MA L L, ZHU M, GAI J W, et al. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential[J]. J Nanobiotechnol, 2020, 18(1): 12. DOI:10.1186/s12951-020-0571-2 |
| [12] |
KAUR S, SINGH S P, ELKAHLOUN A G, et al. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells[J]. Matrix Biol, 2014, 37: 49-59. DOI:10.1016/j.matbio.2014.05.007 |
| [13] |
LIU B N, GUO H Z, XU J, et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses[J]. MAbs, 2018, 10(2): 315-324. DOI:10.1080/19420862.2017.1409319 |
| [14] |
ZHANG Z Z, WANG Q X, LIU Q, et al. Dual-locking nanoparticles disrupt the PD-1/PD-L1 pathway for efficient cancer immunotherapy[J]. Adv Mater Weinheim, 2019, 31(51): e1905751. |
| [15] |
RUAN H T, HU Q Y, WEN D, et al. A dual-bioresponsive drug-delivery depot for combination of epigenetic modulation and immune checkpoint blockade[J]. Adv Mater Weinheim, 2019, 31(17): e1806957. |
| [16] |
LI Y, ZHANG M Y, WANG X D, et al. Vaccination with CD47 deficient tumor cells elicits an antitumor immune response in mice[J]. Nat Commun, 2020, 11(1): 581. |
| [17] |
RING N G, HERNDLER-BRANDSTETTER D, WEISKOPF K, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity[J]. Proc Natl Acad Sci U S A, 2017, 114(49): E10578-E10585. |
| [18] |
ZHANG M, HUTTER G, KAHN S A, et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 Polarized macrophages and promotes M1 Polarized macrophages in vivo[J]. PLoS One, 2016, 11(4): e0153550. |
| [19] |
WEISKOPF K. Cancer immunotherapy targeting the CD47/SIRPα axis[J]. Eur J Cancer Oxf Engl, 2017, 76: 100-109. |
| [20] |
RAMESH A, KUMAR S, NGUYEN A, et al. Lipid-based phagocytosis nanoenhancer for macrophage immunotherapy[J]. Nanoscale, 2020, 12(3): 1875-1885. DOI:10.1039/C9NR08670F |
| [21] |
何剑, 廖红伍, 阳学风. ECEL1基因PSCSI-GFP慢病毒载体的构建[J]. 临床肝胆病杂志, 2019, 35(6): 1286-1292. |
| [22] |
SVENDSEN A, KIEFER H V, PEDERSEN H B, et al. Origin of the Intrinsic fluorescence of the green fluorescent protein[J]. J Am Chem Soc, 2017, 139(25): 8766-8771. DOI:10.1021/jacs.7b04987 |
| [23] |
曹慧玲, 朱小飞, 滕凤猛, 等. GFP作为脐带间充质干细胞体内示踪标志物在大鼠脑缺血再灌注损伤中的表达[J]. 国际检验医学杂志, 2017, 38(19): 2688-2689, 2693. |
| [24] |
THOMAS P, SMART T G. HEK293 cell line:A vehicle for the expression of recombinant proteins[J]. J Pharmacol Toxicol Methods, 2005, 51(3): 187-200. DOI:10.1016/j.vascn.2004.08.014 |
| [25] |
华进, 程志彬, 林春霖, 等. 一种高效稳定的磷酸钙转染293T细胞方法的建立及评价[J]. 吉林大学学报(医学版), 2019, 45(5): 1177-1181, 1198. |
 2020, Vol. 46
2020, Vol. 46


