扩展功能
文章信息
- 周东奎, 鲁明骞, 冯雪松, 刘宇飞, 宋浩, 徐亮
- ZHOU Dongkui, LU Mingqian, FENG Xuesong, LIU Yufei, SONG Hao, XU Liang
- 小肠及回盲部滤泡树突状细胞肉瘤1例报告及文献复习
- Follicular dendritic cell sarcoma of small intestine and ileocecal region: A case report and literature review
- 吉林大学学报(医学版), 2020, 46(04): 858-862
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 858-862
- 10.13481/j.1671-587x.20200431
-
文章历史
- 收稿日期: 2019-11-07
2. 三峡大学第一临床医学院湖北省宜昌市中心人民医院病理科, 湖北 宜昌 443000;
3. 三峡大学第一临床医学院湖北省宜昌市中心人民医院放射影像科, 湖北 宜昌 443000;
4. 三峡大学第一临床医学院湖北省宜昌市中心人民医院超声科, 湖北 宜昌 443000
2. Department of Pathology, Yichang Central People's Hospital, Hubei Province, First College of Clinical Medical Science, China Three Gorges University, Yichang 443000, China;
3. Department of Radiology, Yichang Central People's Hospital, Hubei Province, First College of Clinical Medical Science, China Three Gorges University, Yichang 443000, China;
4. Department of Ultrasound, Yichang Central People's Hospital, Hubei Province, First College of Clinical Medical Science, China Three Gorges University, Yichang 443000, China
滤泡树突状肉瘤(follicular dendritic cell sarcoma,FDCS)是一种发生于淋巴结内和(或)结外器官树突状细胞来源的恶性肿瘤[1],临床上比较罕见,最早于1986年由MONDA等[2]命名并报道。小肠及回盲部滤泡树突状肉瘤是一种发生于小肠(结外器官)的树突状细胞来源的恶性肿瘤,近年来国内外对该病的研究报道[3-7]较少。最初大多数学者认为FDCS是一种惰性行为的恶性肿瘤,但近年来的研究[8]表明其属于中度恶性肿瘤。本文作者报道1例小肠及回盲部FDCS患者,收集其临床表现、病理特征、影像学、免疫组织化学和鉴别诊断等临床资料,并结合相关文献进行回顾性分析,以提高临床工作者对FDCS的认识。
1 临床资料 1.1 一般资料患者,女性,61岁,因下腹部隐痛9 d、发现盆腔肿物5 d,于2019年2月12日就诊于本院。入院后查体:生命体征正常,一般情况可,意识清晰,无病容,查体合作。心肺听诊无异常,腹部平软,右侧腹部可触及一包块,双下肢无水肿。专科妇检:外阴(-),阴道畅,内见少量白色分泌物,宫颈光滑、萎缩,前后穹隆变浅,子宫右前方及后方可触及1个直径约10 cm包块,形态不规则,表面呈结节状,固定,与周围脏器边界不清,轻压痛,左侧附件区未触及明显压痛及包块。肛诊:直肠黏膜光滑,子宫后方及直肠前壁可及结节感。其余各系统查体未见明显异常。
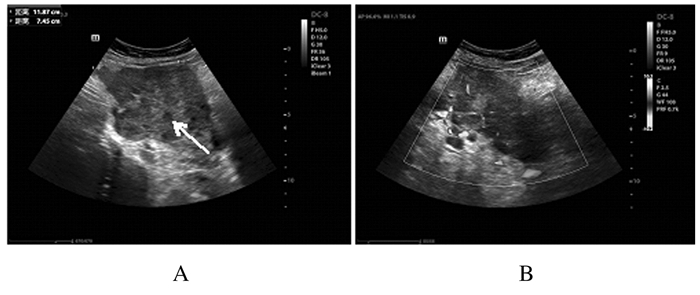
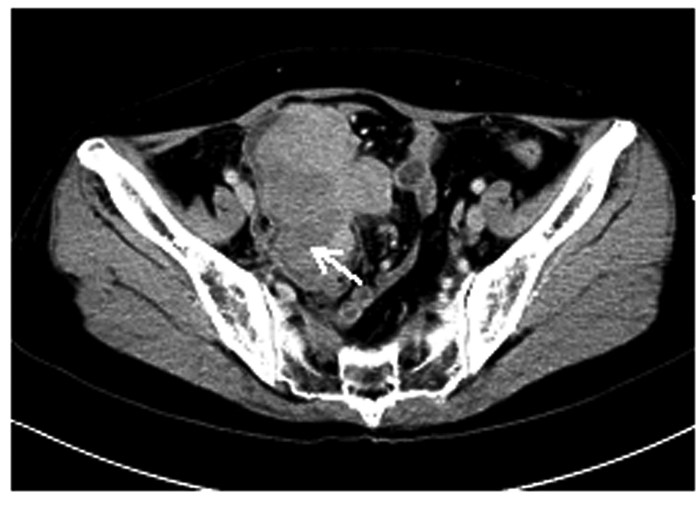
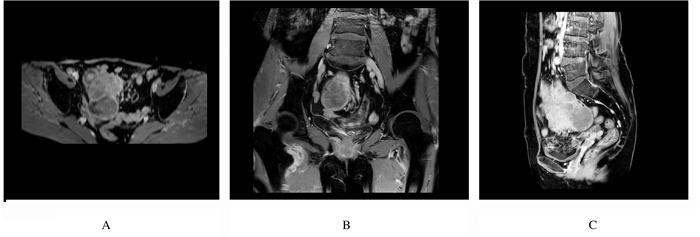
1.2 辅助检查子宫、附件经阴道彩超及腹部彩超检查:盆腔偏右侧低回声包块,考虑恶性肿瘤可能性大,盆腔内多发低回声光团,疑似肿大淋巴结,盆腔积液(图 1);肝内多发低回声光团,疑似转移灶。腹部增强CT检查:考虑右下腹部-盆腔新生物伴周围种植转移,淋巴结增大,请结合临床并进一步检查;肝脏多发斑片状异常强化灶,转移待排,建议复查(图 2)。肝胆脾胰平扫增强MRI检查:肝脏多发斑片状异常强化灶,肿瘤转移可能性大,建议复查。子宫、附件平扫和增强MRI检查:右侧下腹部-盆腔肿块,回盲区结节,考虑恶性肿瘤(右侧卵巢显示不清,与小肠分界不清),伴周围腹膜、盆腔淋巴结转移,建议进一步检查;双侧股骨颈周围软组织内水肿、右侧臀肌肌间隙内积液,转移待排,盆腔积液(图 3)。
|
| Note: Arrow referred to a hypoechoic mass; A:Solid hypoechoic mass on right side of pelvis; B:Abundant blood flow signals in mass. 图 1 FDCS患者术前子宫和附件超声影像 Fig. 1 Ultrasound images of uterus and appendages of FDCS patient before operation |
|
|
|
| Note:Arrow indicated neoplasm in abdominal cavity. 图 2 FDCS患者术前腹部增强CT影像 Fig. 2 Enhanced CT image of abdomen of FDCS patient before operation |
|
|
|
| A:Transverse view; B:Coronal view; C:Sagittal view. 图 3 FDCS患者术前子宫和附件增强MRI影像 Fig. 3 Enhanced MRI images of uterus and appendages of FDCS patient before operation |
|
|
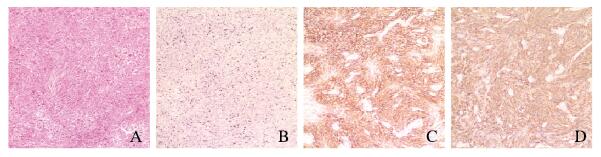
入院后1周,患者于全麻下行腹腔镜探查术+左侧输卵管切除术+腹膜活检术+开腹盆腔肿瘤减灭术+肠切除术+肠吻合术+大网膜切除术。术后病理检查结果:①小肠及回盲部FDCS,小肠断端、结肠断端及阑尾未见肿瘤累犯,肠周淋巴结未见肿瘤转移(0/7)。送检小肠及回盲部肿物组织,于肠管浆膜面系膜组织见灰白灰红色结节数十枚,镜下见肿瘤细胞呈梭形或卵圆形,排列呈束状、漩涡状,单个瘤细胞界限清楚,胞质丰富嗜酸性;核卵圆形或长梭形,染色质空泡状,可见双核或多核瘤巨细胞;核分裂像 < 10个/HPF,肿瘤细胞周围可见较多小淋巴细胞聚集。见图 4A(插页十)。②送检乙状结肠系膜组织、子宫腹壁病灶及盆壁病灶,镜下见均呈FDCS病理形态表现。③送检左侧输卵管及大网膜未见肿瘤累犯。免疫组织化学结果:CD117(-),DOG-1(-),CD34(-),S-100(-),PCK(AE1/AE3)(-),CD3(淋巴细胞+),Ki-67(阳性率30%),Vimentin(+),CD68(组织细胞+),CD20(散在+),CD21(+),ALK(1A4)(-),CD30(-),SMA(-),Desmin(-),CD23(+),HMB-45(-),Melan-A(-),CD35(+),D2-40(+),Inhibin-α(-)。见图 4B~D(插页十)。分子病理结果:EBER-CISH(-)。术后患者恢复尚可,未接受放、化疗等辅助治疗,于2019年3月1日好转出院。
|
|
A: HE staining; B: Ki-67 positive; C:CD21 positive; D:CD23 positive. A: HE staining; B: Ki-67 positive; C:CD21 positive; D:CD23 positive. 图 4 FDCS患者肿瘤组织病理形态表现(A)和免疫组织化学染色结果(B~D)( × 100) Fig. 4 Pathomorphology of tumor tissue(A) and immunohistochemistry staining results(B- D)of FDCS patient( × 100) |
|
|
FDCS是一种临床上少见的恶性肿瘤,好发于颈部和腋窝淋巴结,淋巴结外发病罕见,可见于扁桃体、肝脏、胰腺和腹膜等部位[9],小肠FDCS的报道尤为罕见。FDCS的临床表现无特异性,其确诊主要依靠病理诊断及免疫组织化学检查[10]。小肠FDCS临床表现也不明显,多表现为盆腔逐渐增大的肿物,患者常因肿物压迫周围器官组织引起疼痛、里急后重和腹胀等症状就诊。
FDCS的发病机制目前尚不明确,但近年来关于FDCS的报道逐渐增多,FDCS的病因及发病机制仍有待进一步研究。部分研究[11-12]显示:FDCS与血管透明型Castleman病(hyaline vasculartypeCastleman’ s disease,HVCD)有关,推测FDCS可能通过血管内皮细胞生长因子来刺激滤泡树突状细胞的增生而发生。SANDER等[13]对1例脾脏FDCS进行了细胞遗传表型分析,发现多个染色体位置异常,并指出染色体XP的缺失在FDCS的发生发展过程中起到了重要作用。通过从基因层面对FDCS的深一步研究,将有助于从分子生物学的角度更好地认识FDCS,也为进一步研究FDCS的发病机制提供更多的理论依据。
FDCS患者的临床症状表现不明显,不同年龄均可发病,平均发病年龄约为46岁,发病人群无明显性别差异。不同部位的FDCS临床表现各异,多以各发病部位局部增大的无痛性肿物为主,全身症状多为发热和体质量降低等[14]。侯佳影等[10]报道1例右颈部滤泡树突状细胞肉瘤,因“发现右颈部无痛性肿物缓慢增大”而就诊;柴晓菲等[15]报道1例结肠滤泡树突状细胞肉瘤,体检时左侧腹部可触及1个大小约10 cm×18 cm的肿块,质硬,活动度差,轻压痛。本例患者因下腹隐痛9d、发现盆腔肿物5d而入院,符合临床报道的FDCS的一般临床表现。对于FDCS的诊断,B超、CT、MRI、病理学和免疫组织化学是主要的检查手段。影像诊断主要表现为局部增大的肿物,因其特异性较差,其诊断主要依据为肿瘤组织的病理特点及免疫组织化学特异性标志。FDCS病理特征:肿瘤界限多清楚,光学显微镜下肿瘤细胞呈束状、席纹状、漩涡状和弥漫性生长方式;肿瘤细胞呈梭性、卵圆性,胞质轻度嗜酸性,核染色质细而分散,呈空泡状或者点彩状,小淋巴细胞散在分布于整个肿瘤内,并可见大量淋巴细胞围绕血管周围形成轴套状结构;免疫组织化学染色:CD21、CD23和CD35是FDCS最常见的免疫标志物,特异性强、阳性率高。本例FDCS发生于小肠,彩超提示盆腔偏右实质低回声病灶伴盆腹腔及肝内多发转移,全腹CT提示盆腔右侧多发实性包块,考虑右侧卵巢肿瘤并盆腔种植可能,肿瘤组织病理表现和免疫组织化学染色结果符合FDCS病理特征,以上结果均支持患者小肠及回盲部FDCS的诊断。
FDCS在临床上极易被误诊,鉴别诊断主要根据肿瘤所处部位不同而异,其主要鉴别的肿瘤有:①炎性肌纤维母细胞肿瘤。发生于肝脏的FDCS更需与之鉴别,炎性肌纤维母细胞肿瘤主要表现为明显炎症背景下肿瘤细胞稀疏散在,肿瘤细胞S-腺苷蛋氨酸(SAM)阳性,可表达结蛋白(desmin),约50%患者的间变性淋巴瘤激酶(anaplastic lymphoma kinase,ALK)阳性,GO等[16]研究显示:癌基因BRAF突变可能有助于鉴别炎性肌纤维母细胞肿瘤与炎性假瘤样FDCS。②胰腺实性假乳头状瘤。此病主要好发于年轻女性,增强扫描实性部分呈渐进性强化,肿瘤与周围组织边界清楚,其内可见出血及坏死,通过影像学可初步鉴别,若行病理学及免疫组织化学检测,则二者更易鉴别。③腹腔胃肠间质瘤(gastrointestinal stromal tumor,GIST)。发生在胃肠道的FDCS需与GIST相鉴别,GIST肿瘤细胞表达CD34、CD117和DOG1,不表达CD21、CD35,而FDCS常表达CD21、CD23及CD35,二者可以通过免疫组织化学表达的不同免疫标志物来进行鉴别。④胸腺瘤。FDCS发生于纵膈时需与胸腺瘤进行鉴别,胸腺瘤p53和角蛋白阳性,不表达FDCS标志物。此外,FDCS还需与淋巴瘤、肉瘤样癌、异位转移癌、朗格汉斯细胞组织细胞增生症和恶性纤维组织细胞瘤等相鉴别。FDCS影像表现特异性较差,往往显示局部增大的肿块,所以未经过病理学和免疫组织化学等检查,很难早期做出FDCS的诊断,这也是FDCS容易误诊的原因。
因FDCS发病率较低,临床上报道的病例数较少,到目前为止,尚无统一的标准治疗方法,单纯的手术对短期局部病灶的清除取得了非常满意的效果,但局部复发和远处转移发生率较高[17-18]。陈红敏等[19]研究显示:如果术后给予放化疗等辅助治疗,FDCS患者术后复发和远处转移可有效降低,无病生存期得到延长。FONSECA等[20]报道1例FDCS使用CHOP方案化疗后肿瘤疗效评价为部分缓解(partial remission,PR),继续使用DHAP方案后肿瘤体积进一步缩小,说明化疗有效。有些学者还尝试过其他的化疗方案,包括CHOP、DHAP和ABVD等化疗方案,但疗效有限,其远期疗效仍不理想。PANG等[21]研究显示:头颈部FDCS患者术后联合放疗比单纯手术治疗患者术后无病生存率明显提高。SPATOLA等[22]报道1例FDCS患者经过放疗后复发间隔时间达到了8年。从上述报道可以看出,手术治疗及放、化疗是目前临床上FDCS患者主要的治疗方法。
综上所述,FDCS是一种临床上罕见的恶性肿瘤,来源于小肠及回盲部的FDCS更为罕见,其确诊主要依靠病理检查和免疫组织化学染色,其治疗方法也需要更多的循证医学证据去指导临床。随着对发生于各部位FDCS报道的增多及研究的深入,将会更加完善对FDCS的认识。
| [1] |
GEERTS A, LAGAE E, DHAENE K, et al. Metastatic follicular dendritic cell sarcoma of the stomach:a case report and review of the literature[J]. Acta Gastroenterol Belg, 2004, 67(2): 223-227. |
| [2] |
MONDA L, WARNKE R, ROSAI J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation:a report of 4 cases[J]. Am J Pathol, 1986, 122(3): 562-572. |
| [3] |
HOLLOWOOD K, STAMP G, ZOUVANI I, et al. Extranodal follicular dendritic cell sarcoma of the gastrointestinal tract. Morphologic, immunohistochemical and ultrastructural analysis of two cases[J]. Am J Clin Pathol, 1995, 103(1): 90-97. DOI:10.1093/ajcp/103.1.90 |
| [4] |
LOO C K, HENDERSON C, ROGAN K. Intraabdominal follicular dendritic cell sarcoma:report of a case with fine needle aspiration findings[J]. Acta Cytol, 2001, 45(6): 999-1004. DOI:10.1159/000328378 |
| [5] |
AGAIMY A, WÜNSCH P H. Follicular dendritic cell tumor of the gastrointestinal tract:Report of a rare neoplasm and literature review[J]. Pathol Res Pract, 2006, 202(7): 541-548. DOI:10.1016/j.prp.2006.01.013 |
| [6] |
YAMADA Y, HAGA H, HERNANDEZ M, et al. Follicular dendritic cell sarcoma of small intestine with aberrant T-cell marker expression[J]. Pathol Int, 2009, 59(11): 809-812. DOI:10.1111/j.1440-1827.2009.02449.x |
| [7] |
ZHU H Y, CHEN M, DU Y Q. Follicular dendritic cell sarcoma of the small intestine detected by double-balloon enteroscopy[J]. Dig Endosc, 2017, 29(6): 725-726. DOI:10.1111/den.12896 |
| [8] |
PURKAIT S, MALLICK S, JOSHI P P, et al. Retroperitoneal and mediastinal follicular dendritic cell sarcoma:report of 3 cases with review of literature[J]. Hematol Oncol, 2017, 35(3): 374-379. DOI:10.1002/hon.2275 |
| [9] |
LEE B E, KORST R J, TASKIN M. Right pneumonectomy for resection of a posterior mediastinal follicular dendritic cell sarcoma arising from Castleman's disease[J]. Ann Thorac Surg, 2014, 97(4): e101-e103. DOI:10.1016/j.athoracsur.2013.11.081 |
| [10] |
侯佳影, 邹泓, 刘清华. 右颈部滤泡树突细胞肉瘤1例报道并文献复习[J]. 临床与病理杂志, 2018, 38(8): 1802-1806. |
| [11] |
FEIN A S, TREJO BITTAR H E, SHENDE M R, et al. Castleman disease presenting with pseudotumour cerebri and myasthenia gravis:a case report and literature review[J]. Neuroophthalmology, 2019, 43(3): 185-191. DOI:10.1080/01658107.2018.1484932 |
| [12] |
WANG T, LIU X H, YU Y H. Primary retroperitoneal Castleman disease complicated with follicular dendritic cell sarcoma:report of a case[J]. Chin J Pathol, 2018, 47(7): 557-558. |
| [13] |
SANDER B, MIDDEL P, GUNAWAN B, et al. Follicular dendritic cell sarcoma of the spleen[J]. Hum Pathol, 2007, 38(4): 668-672. DOI:10.1016/j.humpath.2006.08.030 |
| [14] |
HWANG Y Y, CHAN J C, TRENDELL-SMITH N J, et al. Recalcitrant paraneoplastic pemphigus associated with follicular dendritic cell sarcoma:response to prolonged rituximab and ciclosporin therapy[J]. Intern Med J, 2014, 44(11): 1145-1146. DOI:10.1111/imj.12576 |
| [15] |
柴晓菲, 王娟, 夏庆欣, 等. 结肠滤泡树突状细胞肉瘤伴多发转移临床病理分析[J]. 肿瘤基础与临床, 2017, 30(3): 209-212. |
| [16] |
GO H, JEON Y K, HUH J, et al. Frequent detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms[J]. Histopathology, 2014, 65(2): 261-272. |
| [17] |
SAHAY A, BAL M, PATIL A, et al. Follicular dendritic cell sarcoma of the larynx:apropos a rare case with review of the literature[J]. Turk Patoloji Derg, 2019, 35(3): 254-257. |
| [18] |
CHIN K M, HO W Y, LIM K, et al. Follicular dendritic cell sarcoma of the liver with metachronous small bowel and splenic metastases:a case report and literature review[J]. Hepatobiliary Surg Nutr, 2017, 6(3): 179-189. |
| [19] |
陈红敏, 丁云丽, 徐佳. 滤泡性树突状细胞肉瘤研究进展[J]. 浙江创伤外科, 2015, 20(6): 1257-1259. |
| [20] |
FONSECA R, YAMAKAWA M, NAKAMURA S, et al. Follicular dendritic cell sarcoma and interdigitating reticulum cell sarcoma:a review[J]. Am J Hematol, 1998, 59(2): 161-167. DOI:10.1002/(SICI)1096-8652(199810)59:2<161::AID-AJH10>3.0.CO;2-C |
| [21] |
PANG J, MYDLARZ W K, GOOI Z, et al. Follicular dendritic cell sarcoma of the head and neck:Case report, literature review, and pooled analysis of 97 cases[J]. Head Neck, 2016, 38(Suppl 1): E2241-E2249. |
| [22] |
SPATOLA C, MIGLIORE M, EMANUELE L R, et al. Follicular dendritic cell sarcoma of mediastinum:a key role of radiotherapy in a multidisciplinary approach[J]. Future Oncol, 2015, 11(24 Suppl): 57-61. |
 2020, Vol. 46
2020, Vol. 46


