扩展功能
文章信息
- 秦巧红, 张楠, 赵书君, 李红雨
- QIN Qiaohong, ZHANG Nan, ZHAO Shujun, LI Hongyu
- miR-216a-5p和WASL在子宫内膜癌组织中的表达及其调控子宫内膜癌细胞增殖、迁移和侵袭的分子机制
- Expressions of miR-216a-5p and WASL in endometrial carcinoma tissue and their molecular mechanisms of regulating proliferation, migration and invasion of endometrial carcinoma cells
- 吉林大学学报(医学版), 2020, 46(04): 844-850
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 844-850
- 10.13481/j.1671-587x.20200429
-
文章历史
- 收稿日期: 2019-07-17
子宫内膜癌是女性最常见的恶性肿瘤之一,其发病率居女性生殖系统肿瘤的第4位,并且有逐年上升的趋势,且发病人群呈年轻化趋势[1]。虽然通过早期的诊断和规范化治疗,子宫内膜癌患者5年生存率逐年升高,但目前对子宫内膜癌发生发展的分子机制认识不足,晚期子宫内膜癌患者的治疗效果仍不理想[2-3]。因此,研究子宫内膜癌的分子机制对于子宫内膜癌的早期诊断及治疗均具有非常重要的意义。
微小RNA(microRNA,miRNA)是一类长度为18~25 nt的高度保守的非编码小RNA,可组装成RNA诱导的沉默复合物(RNA-induced silencing complex,RISC),进而通过翻译抑制或mRNA裂解作用于特定的基因靶点,在细胞分化、转移、发育和凋亡中发挥重要作用[4-5]。miR-216家族是miRNA的一种,周胤健等[6]在研究子宫内膜癌miRNA表达谱时发现:miR-216b在子宫内膜癌中低表达,但miR-216a-5p在子宫内膜癌中的作用机制尚不清楚。湿疹血小板减少伴免疫缺陷综合征样蛋白(Wiskott-Aldrich syndrome likeprotein,WASL)属于湿疹血小板减少伴免疫缺陷综合征蛋白(Wiskott-Aldrich syndrome protein,WASP)家族蛋白之一,参与调控肌动蛋白相关蛋白2/3(actin-related protein 2/3, ARP 2/3)相关通路[7-9]并参与多种癌症的发生发展。
miR-216a-5p在不同癌症中的作用与其靶基因有关,关于其在子宫内膜癌中的靶基因及其影响子宫内膜癌的机制还有待进一步研究。本研究探讨miR-216a-5p与WASL是否存在靶向关系,并进一步分析miR-216a-5p靶向WASL对子宫内膜癌细胞增殖、迁移和侵袭的影响,以期为子宫内膜癌的预防和治疗提供新的靶点和方向。
1 材料与方法 1.1 细胞、组织标本和主要试剂子宫内膜癌HEC-1-B细胞购自中国科学院(上海)生物化学和细胞生物学研究所。组织标本为本院2017年8月—2018年5月手术切除的47例子宫内膜癌患者癌组织及癌旁组织,于-80 ℃条件下保存。本研究经本医院医学伦理委员会批准,所有患者及家属均签署知情同意书。胎牛血清、胰蛋白酶和改良Eagle培养基购自美国Gibco公司,miR-216a-5p模拟物(miR-216a-5p)、miR-216a-5p抑制物(anti-miR-216a-5p)、模拟物阴性对照(miR-con)、抑制物阴性对照(anti-miR-con)和小干扰RNA(siRNA)片段购自广州锐博生物科技有限公司,pcDNA3.1载体和LipofectamineTM 2000转染试剂购自美国Invitrogen公司,psiCHECK2载体购自美国Promega公司,MTT试剂盒、DMSO、BCA试剂盒和PBS缓冲液购自美国Sigma公司,双荧光素酶报告基因检测试剂盒购自北京白奥莱博科技有限公司,TRIzol试剂、逆转录试剂盒和荧光定量试剂盒购自日本TaKaRa公司,RIPA蛋白裂解液购自上海碧云天生物技术有限公司,抗体购自北京博奥森生物科技有限公司。Transwell小室(8.0 μm孔径)和基质胶购于美国BD公司,实时荧光定量PCR仪购自美国Applied Biosystems公司,倒置荧光显微镜购自日本Olympus公司,多功能酶标仪购自德国BMG LabTech公司。
1.2 细胞培养和转染子宫内膜癌HEC-1-B细胞采用含10%胎牛血清的改良Eagle培养基,置于37 ℃、5% CO2培养箱中培养,每2 d更换1次培养基。选取处于对数生长期的细胞用0.25 %胰蛋白酶进行消化,然后接种于96孔板中,待细胞融合至70%~80%时,分为miR-216a-5p组、miR-con组、anti-miR-con组、anti-miR-216a-5p组、沉默对照(si-con)组、沉默WASL的siRNA(si-WASL)组和共转染miR-216a-5p+pcDNA组及miR-216a-5p+pcDNA-WASL组,将上述各组不同转染质粒与不完全培养基混合后,加入各组HEC-1-B细胞中,滴加至24孔板,37℃、5%CO2培养箱中培养48 h。参照LipofectamineTM 2000试剂盒说明书进行转染。
1.3 逆转录实时荧光定量PCR(Real-time RT-qPCR)法检测组织和细胞中miR-216a-5p和WASL mRNA表达水平收集适量子宫内膜癌组织及癌旁组织和处于对数生长期的各组细胞,充分研磨后加入TRIzol裂解细胞,参照RNA抽提试剂盒说明书进行操作。检测RNA纯度和浓度合格后,使用逆转录试剂盒合成cDNA。扩增条件:94℃、30 s,64℃、30 s,72 ℃、40 s,共34个循环;72 ℃、8 min。以β-actin和U6为内参,采用2-△△Ct法计算miR-216a-5p和WASL mRNA表达水平。WASL引物:F 5′-TCCACACAACTCAGGTCCTC-3′,R 5′-GTGGTGTAGACTCTTGGCCA-3′;β-actin引物:F 5′-GGACCTGACTGACTACCTC -3′,R 5′- TACTCCTGCTTGCTGAT-3′;miR-216a-5p引物:F 5′-TGTCGCAAATCTCTGCAGG,R 5′-CAGAGCAGGGTCCGAGGTA;U6引物:F 5′-CTCGCTTCGGCAGCACA-3′,R 5′-AACGCTTCACGAATTTGCGT-3′。
1.4 靶基因预测和验证通过生物信息学在线靶基因预测网站Target genes(http://www.Targetscan.org/)预测miR-216a-5p的靶基因,并确定其与靶基因的结合位点。
1.5 Western blotting法检测组织和细胞中WASL蛋白表达水平收集适量子宫内膜癌组织及癌旁组织和处于对数生长期的各组细胞,加入RIPA裂解液裂解细胞后离心提取总蛋白,采用BCA试剂盒参照说明书对蛋白进行定量。蛋白样品SDS-PAGE电泳后转至PVDF膜上,封闭2 h,一抗4 ℃孵育过夜,TBST洗膜后二抗(辣根过氧化物酶标记)室温孵育1 h,TBST洗膜后加入显影混合液显影。以β-actin为内参,应用凝胶成像系统分析蛋白条带的灰度值,以目的蛋白条带灰度值/β-actin蛋白条带灰度值比值代表蛋白表达水平。
1.6 MTT法检测细胞增殖活性收集适量培养24、48和72 h的各组细胞,分别加入20 μLMTT溶液,孵育4 h后弃掉上清,加入150 μL DMSO振荡反应10 min。采用酶标仪于490 nm波长下检测细胞吸光度(A)值,以A值代表各组细胞增殖活性。
1.7 Transwell实验检测各组细胞中迁移和侵袭细胞数收集适量处于对数生长期的各组细胞,胰酶消化后重悬细胞,调整细胞浓度为1×105 mL-1。取400 μL细胞悬液于Transwell小室的上室内,取600 μL培养基于下室内,37℃、5% CO2条件下培养过夜。取出小室,弃掉培养基,用棉签小心地擦掉上室内的细胞后用PBS冲洗3次,加入4 %多聚甲醛固定30 min,0.1%结晶紫染色20 min,PBS冲洗3次。显微镜下随机选取3个视野拍照,计算小室下表面附着的细胞数,即迁移细胞数。
取100 μL冰上融化的基质胶加入300 μL预冷的培养基,混合均匀,加入小室的上室内,使其均匀覆盖,室温干燥备用。调整细胞密度后参照上述细胞迁移实验过程进行,显微镜下随机选取3个视野拍照,计算小室下表面附着的细胞数,即侵袭细胞数。
1.8 双荧光素酶报告基因实验取适量对数生长期miR-Con和miR-216a-5p组细胞,psiCHECK2载体以萤火虫荧光素酶活性为内参,野生型WASL基因表达载体psiCHECK2-WASL-3′UTR WT(WT-WASL)和突变型WASL基因表达载体psiCHECK2-WASL -3′UTR MUT (MUT-WASL)的表达为对照,参照双荧光素酶报告基因检测试剂盒说明书进行操作,首先加入1×Passive Lysis Buffer置于4℃保持20 min后得到细胞裂解液,然后吸取40 μL细胞裂解液与20 μL萤火虫荧光素酶缓冲液,混合均匀,检测萤火虫荧光酶荧光值;再加入20 μL海肾荧光素酶缓冲液,混合均匀,检测海肾荧光酶荧光值。
1.9 统计学分析采用SPSS 20.0统计软件进行统计学分析。子宫内膜癌组织和癌旁组织中miR-216a-5p和WASL mRNA及WASL蛋白表达水平,各组HEC-1-B细胞中miR-216a-5p和WASL mRNA及WASL蛋白表达水平,各组细胞增殖活性、迁移细胞数和侵袭细胞数,采用K-S检验进行正态性检验,均符合正态分布,均以x±s表示,2组间样本均数比较采用t检验或配对t检验,多组间样本均数比较采用单因素方差分析,组间样本均数两两比较采用SNK-q检验。子宫内膜癌组织中miR-216a-5p与WASL mRNA表达水平的相关性分析采用Pearson相关性分析。检验水准为α=0.05。
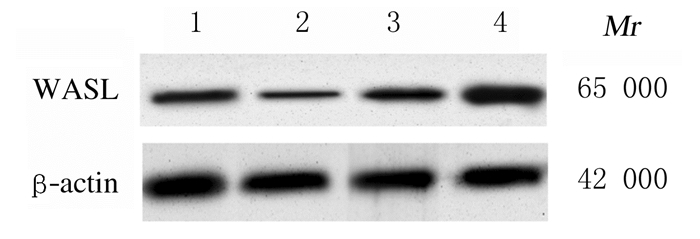
2 结果 2.1 子宫内膜癌组织和癌旁组织中miR-216a-5p和WASL mRNA及蛋白表达水平Real-Time RT-qPCR检测结果显示:与癌旁组织比较,子宫内膜癌组织中miR-216a-5p表达水平明显降低(P < 0.05),WASL mRNA表达水平明显升高(P < 0.01)。Western blotting法检测结果显示:与癌旁组织比较,子宫内膜癌组织中WASL蛋白表达水平明显升高(P < 0.05)。见图 1和表 1。子宫内膜癌组织中miR-216a-5p表达水平与WASLmRNA表达水平呈负相关关系(r=-0.317,P < 0.01)。见图 2。
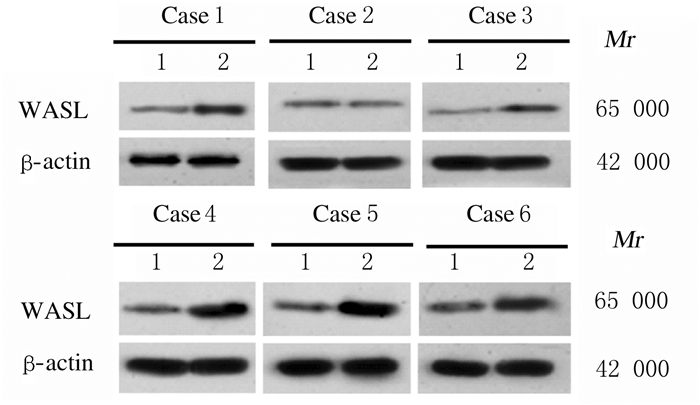
|
| Lane 1:Adjacent tissue; Lane 2:Endometrial carcinoma tissue. 图 1 Western blotting法检测子宫内膜癌组织和癌旁组织中WASL蛋白表达电泳图 Fig. 1 Electrophoregram of expressions of WASL protein in endometrial carcinoma tissue and adjacent tissue detected by Western blotting method |
|
|
| (n=47, x±s) | |||||||||||||||||||||||||||||
| Group | miR-216a-5p | WASL mRNA | WASL protein | ||||||||||||||||||||||||||
| Adjacent tissue | 1.04±0.27 | 0.97±0.22 | 0.43±0.12 | ||||||||||||||||||||||||||
| Endometrial | 0.68±0.26* | 1.97±0.86* | 0.84±0.27* | ||||||||||||||||||||||||||
| carcinoma tissue | |||||||||||||||||||||||||||||
| * P < 0.05 compared with adjacent tissue. | |||||||||||||||||||||||||||||
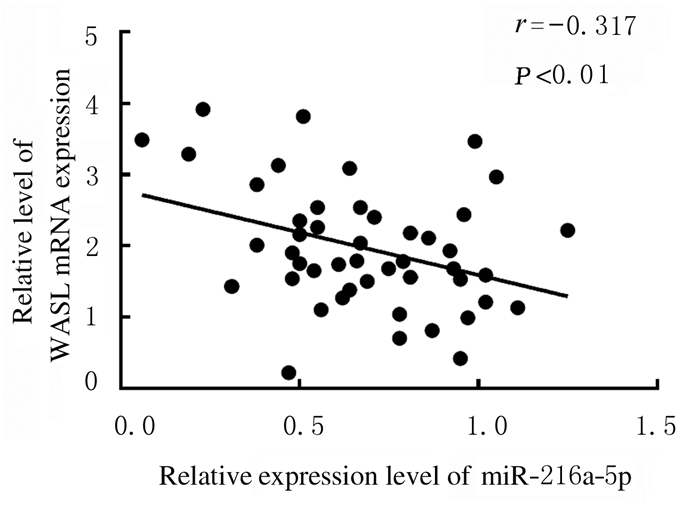
|
| 图 2 子宫内膜癌组织中miR-216a-5p与WASLmRNA表达水平相关性分析 Fig. 2 Correlation analysis on expression levels of miR-216a-5p and WASL mRNA in endometrial carcinoma tissue |
|
|
与miR-con组比较,miR-216a-5p组细胞中WASL蛋白表达水平、细胞增殖活性、迁移细胞数和侵袭细胞数均明显降低(P < 0.05)。与si-con组比较,si-WASL组细胞中WASL蛋白表达水平、细胞增殖活性、迁移细胞数和侵袭细胞数进一步降低(P < 0.05)。见图 3和图 4(插页十)及表 2。
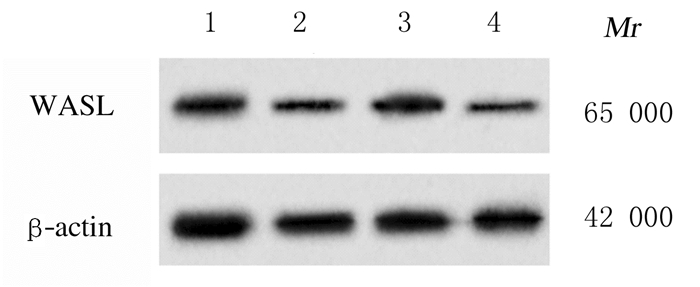
|
| Lane 1: MiR-con group; Lane 2: MiR-216a-5p group; Lane 3: Si-con group; Lane 4: Si-WASL group. 图 3 Western blotting法检测各组细胞中WASL蛋白表达电泳图 Fig. 3 Electrophoregram of expressions of WASL protein in cells in various groups detected by Western blotting method |
|
|
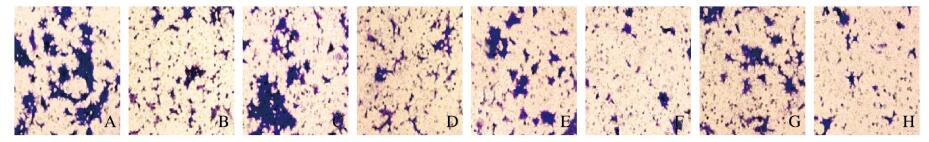
|
|
A-D: Migration; E-H: Invasion; A. E: miR-con group; B, F :miR-216a-5p group; C, G:si-con group; D, H: si-WASL group. (seen on page 847 in paragraph) 图 4 Transwell小室实验检测各组HEC-1-B细胞迁移和侵袭情况(结晶紫, ×200) Fig. 4 Migration and invasion of HEC-1-B cells in various groups detected by Transwell chamber experiment(Crystal violet, ×200) |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | WASL protein | Proliferation activity | Number of migrated cells | Number of invasive cells | |||||||||||||||||||||||||
| (t/h) 24 | 48 | 72 | |||||||||||||||||||||||||||
| MiR-con | 0.73±0.08 | 0.38±0.04 | 0.67±0.06 | 1.12±0.09 | 93.26±8.46 | 47.24±5.26 | |||||||||||||||||||||||
| MiR-216a-5p | 0.31±0.04* | 0.34±0.03* | 0.47±0.05* | 0.77±0.08* | 35.13±4.72* | 19.35±3.37* | |||||||||||||||||||||||
| Si-con | 0.81±0.11 | 0.39±0.05 | 0.71±0.07 | 1.22±0.12 | 113.08±10.23 | 54.86±5.68 | |||||||||||||||||||||||
| Si-WASL | 0.22±0.04*△ | 0.33±0.04*△ | 0.41±0.04*△ | 0.69±0.08*△ | 27.31±4.25*△ | 12.18±2.56*△ | |||||||||||||||||||||||
| F | 145.037 | 4.728 | 268.201 | 68.601 | 299.427 | 195.166 | |||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |||||||||||||||||||||||
| * P < 0.05 compared with miR-con group; △ P < 0.05 compared with si-con group. | |||||||||||||||||||||||||||||
TargetScan数据库预测结果显示:WASL与miR-216a-5p之间存在结合位点(图 5)。双荧光素酶报告基因检测结果显示:转染野生型WASL基因表达载体WT-WASL后,与miR-con组比较,miR-216a-5p组WT-WASL载体的细胞荧光活性明显降低(P < 0.01);而转染突变型WASL基因表达载体MUT-WASL后,与miR-con组比较,miR-216a-5p组MUT-WASL载体的细胞荧光活性差异无统计学意义(P>0.05)。见表 3。Western blotting法检测结果显示:与miR-con组比较,miR-216a-5p组细胞中WASL蛋白表达水平明显降低(P < 0.05);与anti-miR-con组比较,anti-miR-216a-5p组细胞中WASL蛋白表达水平明显升高(P < 0.05),表明miR-216a-5p可靶向调控WASL的表达。见图 6和表 4。
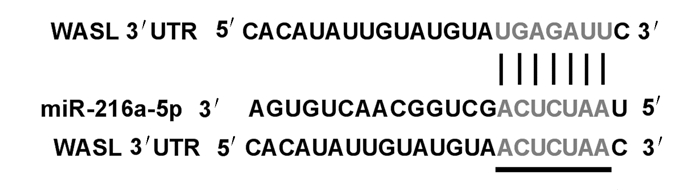
|
| 图 5 miR-216a-5p与WASL互补的核苷酸序列信息 Fig. 5 Complementary nucleotide sequence information of miR-216a-5p and WASL |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | Fluorescence activity | ||||||||||||||||||||||||||||
| WT-WASL | MUT-WASL | ||||||||||||||||||||||||||||
| MiR-con | 1.00±0.11 | 1.41±0.18 | |||||||||||||||||||||||||||
| MiR-216a-5p | 0.54±0.07* | 1.29±0.15 | |||||||||||||||||||||||||||
| * P < 0.05 compared with miR-con group. | |||||||||||||||||||||||||||||
|
| Lane 1:MiR-con group; Lane 2:MiR-216a-5p group; Lane 3:Anti-miR-con group; Lane 4:Anti-miR-216a-5p group. 图 6 Western blotting法检测各组子宫内膜癌细胞中WASL蛋白表达电泳图 Fig. 6 Electrophoregram of expressions of WASL protein in endometrial carcinoma cells detected by Western blotting method |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | WASL protein | ||||||||||||||||||||||||||||
| MiR-con | 0.61±0.06 | ||||||||||||||||||||||||||||
| MiR-216a-5p | 0.24±0.04* | ||||||||||||||||||||||||||||
| Anti-miR-con | 0.67±0.07 | ||||||||||||||||||||||||||||
| Anti-miR-216a-5p | 1.14±0.09△ | ||||||||||||||||||||||||||||
| F | 269.868 | ||||||||||||||||||||||||||||
| P | < 0.01 | ||||||||||||||||||||||||||||
| * P < 0.05 compared with miR-con group;△ P < 0.05 compared with anti-miR-con group. | |||||||||||||||||||||||||||||
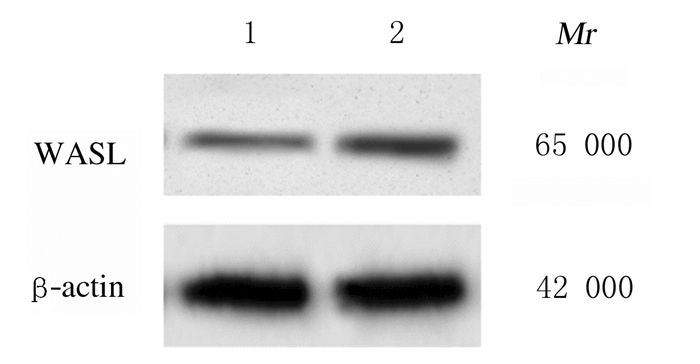
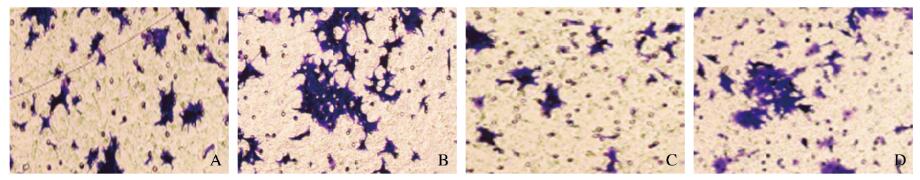
与miR-216a-5p + pcDNA组比较,miR-216a-5p + pcDNA-WASL组细胞中WASL蛋白表达水平明显升高(P < 0.01),细胞增殖活性、迁移细胞数和侵袭细胞数明显增加(P < 0.05或P < 0.01),表明过表达WASL可逆转miR-216a-5p对子宫内膜癌HEC-1-B细胞增殖活性、迁移和侵袭能力的抑制作用。见图 7和图 8(插页十)及表 5。
|
| Lane 1:MiR-216a-5p + pcDNA group; Lane 2:MiR-216a-5p + pcDNA-WASL group. 图 7 Western blotting法检测共转染组细胞中WASL蛋白表达电泳图 Fig. 7 Electrophoregram of expressions of WASL protein in cells in miR-216a-5p+pcDNA group and miR-216a-5p+pcDNA-WASL group detected by Western blotting method |
|
|
|
|
A, B: Migration; C, D: Invasion. (seen on page 848 in paragraph) 图 8 Transwell小室实验检测miR-216a-5p+ peDNA组(A, C)和miR-216a-5p+ peDNA-WASL组(B, D) HEC-1-B细胞迁移和侵袭情况(结晶紫, ×200) Fig. 8 Migration and invasion of HEC-1-B cells in miR-216a-5p + pcDNA group(A, C) and miR-216a-5p + pcDNA-WASL group(B, D) detected by Transwell chamber experiment(Crystal violet, × 200) |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | WASL protein | Proliferation activity | Number of migrated cells | Number of invasive cells | |||||||||||||||||||||||||
| (t/h) 24 | 48 | 72 | |||||||||||||||||||||||||||
| MiR-216a-5p+pcDNA | 0.31±0.05 | 0.33±0.03 | 0.48±0.04 | 0.75±0.08 | 44.65±3.37 | 26.56±3.25 | |||||||||||||||||||||||
| MiR-216a-5p+pcDNA-WASL | 0.48±0.05 | 0.37±0.04 | 0.58±0.08 | 1.08±0.09 | 63.58±7.64 | 39.46±4.28 | |||||||||||||||||||||||
| t | 7.212 | 2.400 | 3.354 | 7.474 | 6.801 | 7.201 | |||||||||||||||||||||||
| P | < 0.01 | 0.03 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |||||||||||||||||||||||
子宫内膜癌是威胁女性生命健康的恶性肿瘤之一,采用传统的手术联合放疗和化疗治疗效果不明显且对人体伤害较大,新型靶向治疗的出现可以在杀死肿瘤细胞的同时减少对正常细胞的损伤,是目前子宫内膜癌防治的研究方向和热点[10]。miRNA表达异常参与多种癌症的进展,影响肿瘤细胞的增殖、分化、转移、侵袭和凋亡[11]。邵焕军等[12]研究显示:膀胱癌组织中miR-216a-5p表达降低,上调miR-216a-5p表达可抑制膀胱癌细胞的增殖并诱导细胞凋亡;ZHANG等[13]研究显示:乳腺癌患者癌组织中miR-216a-5p低表达,通过靶向p21蛋白激活激酶2(p21 protein activated kinase 2,PAK2)调控乳腺癌细胞增殖和转移。在宫颈癌细胞中miR-216a-5p也呈低表达[14],但miR-216a-5p在子宫内膜癌中的作用尚未见报道。本研究结果显示:与癌旁组织比较,miR-216a-5p在子宫内膜癌组织中低表达,WASL mRNA高表达,两者呈负相关关系,表明子宫内膜癌发展过程中miR-216a-5p与WASL mRNA异常表达有关;过表达miR-216a-5p可抑制子宫内膜癌细胞的增殖和细胞迁移侵袭。miR-216a-5p在不同肿瘤中的作用及靶基因不同。本研究中靶基因预测结果显示:miR-216a-5p与WASL之间存在靶向结合位点,进一步将野生型和突变型WASL表达载体与miR-con、miR-216a-5p共转染后检测荧光素酶活性,结果证明WASL是miR-216a-5p的靶基因。
WASL蛋白是调节ARP2/3的信号分子,其介导从细胞表面受体到肌动蛋白细胞骨架的信号,进而通过激活ARP2/3复合物加速肌动蛋白的聚合[15-16]。已有研究[17]表明:癌细胞的细胞膜受体数量、癌细胞形状及癌大小与癌细胞的侵袭能力有关。MORRIS等[18]和WANG等[19]发现:WASL蛋白在结直肠癌肝转移和食管鳞癌组织中均有表达。YANG等[20]研究发现:WASL蛋白在肝癌中广泛存在; PENG等[21]也发现:在葡萄膜黑素瘤组织中WASL高表达,抑制其表达可抑制癌细胞的增殖。本研究结果显示:沉默WASL后子宫内膜癌细胞的增殖活性、迁移细胞数和侵袭细胞数均明显降低,说明WASL与子宫内膜癌的发生有关;进一步将miR-216a-5p和pcDNA-WASL共转染子宫内膜癌细胞,miR-216a-5p对子宫内膜癌细胞的抑制作用得到部分逆转。
综上所述,WASL是miR-216a-5p的靶基因,过表达miR-216a-5p可通过靶向负调控WASL的表达抑制子宫内膜癌细胞的增殖活性、迁移和侵袭能力,进而影响子宫内膜癌的进展。本研究结果为miR-216a-5p靶向防治子宫内膜癌提供了一个新的方向。
| [1] |
TORRE LA, BRAY F, SIEGEL RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2): 87-108. DOI:10.3322/caac.21262 |
| [2] |
VITALE S G, VALENTI G, GULINO F A, et al. Surgical treatment of high stage endometrial cancer:current perspectives[J]. Updates Surg, 2016, 68(2): 149-154. DOI:10.1007/s13304-015-0340-1 |
| [3] |
YANOKURA M, BANNO K, ⅡDA M, et al. MicroRNAS in endometrial cancer:recent advances and potential clinical applications[J]. EXCLI J, 2015, 14: 190-198. |
| [4] |
SIVALINGAM V N, KITSON S, MCVEY R, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer[J]. Br J Cancer, 2016, 114(3): 281-289. |
| [5] |
KIM J, YAO F, XIAO Z N, et al. MicroRNAs and metastasis:small RNAs play big roles[J]. Cancer Metastasis Rev, 2018, 37(1): 5-15. |
| [6] |
周胤健, 杜英, 梅丽娜, 等. 子宫内膜癌microRNA表达谱的初步研究[J]. 中华实验和临床病毒学杂志, 2014, 28(4): 280-282. DOI:10.3760/cma.j.issn.1003-9279.2014.04.013 |
| [7] |
YAMAGUCHI H, MIKI H, TAKENAWA T. Neural Wiskott-Aldrich syndrome protein is involved in hepatocyte growth factor-induced migration, invasion, and tubulogenesis of epithelial cells[J]. Cancer Res, 2002, 62(9): 2503-2509. |
| [8] |
KAWAMURA K, TAKANO K, SUETSUGU S, et al. N-WASP and WAVE2 acting downstream of phosphatidylinositol 3-kinase are required for myogenic cell migration induced by hepatocyte growth factor[J]. J Biol Chem, 2004, 279(52): 54862-54871. DOI:10.1074/jbc.M408057200 |
| [9] |
DONNELLY S K, WEISSWANGE I, ZETTL M, et al. WIP provides an essential link between Nck and N-WASP during Arp2/3-dependent actin polymerization[J]. Curr Biol, 2013, 23(11): 999-1006. DOI:10.1016/j.cub.2013.04.051 |
| [10] |
HILL E K, DIZON D S. Medical therapy of endometrial cancer:current status and promising novel treatments[J]. Drugs, 2012, 72(5): 705-713. |
| [11] |
冯双苗, 杨贺佳, 袁银花, 等. LncRNA CASC2与miR-115-5p的靶向关系及对子宫内膜癌细胞迁移、侵袭能力的影响[J]. 郑州大学学报(医学版), 2020, 55(2): 243-248. |
| [12] |
邵焕军, 赵振伶, 郝丽娜, 等. miR-216a-5p靶向作用于PAK2对膀胱癌细胞增殖和凋亡影响的体外研究[J]. 医学研究杂志, 2018, 47(6): 151-155. |
| [13] |
ZHANG Y, LIN P, ZOU J Y, et al. MiR-216a-5p act as a tumor suppressor, regulating the cell proliferation and metastasis by targeting PAK2 in breast cancer[J]. Eur Rev Med Pharmacol Sci, 2019, 23(6): 2469-2475. |
| [14] |
ZHU H, ZENG Y, ZHOU CC, et al. SNHG16/miR-216-5p/ZEB1 signal pathway contributes to the tumorigenesis of cervical cancer cells[J]. Arch Biochem Biophys, 2018, 637: 1-8. DOI:10.1016/j.abb.2017.11.003 |
| [15] |
UENISHI E, SHIBASAKI T, TAKAHASHI H, et al. Actin dynamics regulated by the balance of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion[J]. J Biol Chem, 2013, 288(36): 25851-25864. DOI:10.1074/jbc.M113.464420 |
| [16] |
ALEKHINA O, BURSTEIN E, BILLADEAU D D. Cellular functions of WASP family proteins at a glance[J]. J Cell Sci, 2017, 130(14): 2235-2241. DOI:10.1242/jcs.199570 |
| [17] |
SCHWICKERT A, WEGHAKE E, BRÜGGEMANN K, et al. MicroRNA miR-142-3p inhibits breast cancer cell invasiveness by synchronous targeting of WASL, integrin alpha V, and additional cytoskeletal elements[J]. PLoS One, 2015, 10(12): e0143993. DOI:10.1371/journal.pone.0143993 |
| [18] |
MORRIS H T, FORT L, SPENCE H J, et al. Loss of N-WASP drives early progression in an Apc model of intestinal tumourigenesis[J]. J Pathol, 2018, 245(3): 337-348. DOI:10.1002/path.5086 |
| [19] |
WANG W S, ZHONG H J, XIAO D W, et al. The expression of CFL1 and N-WASP in esophageal squamous cell carcinoma and its correlation with clinicopathological features[J]. Dis Esophagus, 2010, 23(6): 512-521. DOI:10.1111/j.1442-2050.2009.01035.x |
| [20] |
YANG L Y, TAO Y M, OU D P, et al. Increased expression of Wiskott-Aldrich syndrome protein family verprolin-homologous protein 2 correlated with poor prognosis of hepatocellular carcinoma[J]. Clin Cancer Res, 2006, 12(19): 5673-5679. DOI:10.1158/1078-0432.CCR-06-0022 |
| [21] |
PENG D W, DONG J, ZHAO Y P, et al. MiR-142-3p suppresses uveal melanoma by targeting CDC25C, TGFβR1, GNAQ, WASL, and RAC1[J]. Cancer Manag Res, 2019, 11: 4729-4742. DOI:10.2147/CMAR.S206461 |
 2020, Vol. 46
2020, Vol. 46


