扩展功能
文章信息
- 张倩, 冯晴霞, 周正乙, 李睿祺, 方雷, 杨秋楠, 盛瑜, 卢学春, 杜培革, 安丽萍
- ZHANG Qian, FENG Qingxia, ZHOU Zhengyi, LI Ruiqi, FANG Lei, YANG Qiunan, SHENG Yu, LU Xuechun, DU Peige, AN Liping
- 刺五加苷B对疲劳小鼠学习记忆能力的改善作用及其激活Keap1/Nrf2/ARE信号通路的机制
- Improvement effect of eleutheroside B on learning and memory abilities of fatigue mice and its mechanism of activating Keap1/Nrf2/ARE signaling pathway
- 吉林大学学报(医学版), 2020, 46(04): 771-778
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 771-778
- 10.13481/j.1671-587x.20200417
-
文章历史
- 收稿日期: 2019-12-07
2. 解放军总医院血液科, 北京 100853
2. Department of Hematology, PLA General Hospital, Beijing 100853, China
疲劳是由于长时间运动后造成体力和脑力损耗,从而对人们身体和心理产生影响的一种生理现象[1]。机体的能源物质耗竭且供应不足、自由基积累脂质过氧化反应增强和代谢产物堆积不能及时分解均为产生疲劳的主要原因[2]。长时间运动可导致脑内中枢神经递质发生变化,引起神经系统的机能改变,也会导致机体抗氧化功能减弱,从而影响学习记忆能力[3-4]。
刺五加(Acanthopanax senticosus)系五加科植物的干燥根和根茎,具有抗癌、抗辐射、抗衰老和抗疲劳等功能,其主要的活性单体成分为刺五加苷B(eleutheroside B,ELB)和刺五加苷E(eleutheroside E,ELE),ELB占刺五加总苷的45% [5-8]。ELB是刺五加抗疲劳的主要活性成分,但其抗疲劳的机制尚不明确。研究[9]显示:ELB对PC12细胞具有神经保护作用,可增强其抗氧化能力。有关ELB对疲劳小鼠学习记忆功能影响的研究国内外尚未见报道。本文作者以游泳训练致疲劳的小鼠为模型,通过行为学实验、生化指标检测、HE染色和Western blotting法检测,观察ELB对疲劳小鼠学习记忆功能的影响,并探讨其相关机制,为刺五加活性成分的利用和相关产品的深度开发奠定理论和实践基础。
1 材料与方法 1.1 动物、主要试剂和仪器60只雄性ICR小鼠(吉林省长春市亿斯实验动物技术有限责任公司),动物生产许可证号为SCXK(吉)-2018-0007,体质量为(20±2)g。ELB(ID:QZ3Y-Y3AD;111574-201605,中国食品药品检定研究院),乳酸(lactic acid, LD)试剂盒、乳酸脱氢酶(lactic dehydrogebase, LDH)试剂盒、血尿素氮(blood urea nitrogen, BUN)试剂盒、超氧化物歧化酶(superoxidedismutase, SOD)试剂盒、丙二醛(malondialdehyde, MDA)试剂盒和三磷酸腺苷酶(adenosine triphosphase, ATPase)试剂盒(南京建成生物工程研究院),活性氧簇(reactive oxygen, ROS)试剂盒、谷氨酸(glutamic acid, GLU)试剂盒、内皮型一氧化氮合酶(endothelial nitric oxide synthase, eNOS)试剂盒和γ氨基丁酸(γ-aminobutyric acid, GABA)试剂盒(上海酶联生物科技有限公司),BCA蛋白定量测定试剂盒、Kelch样环氧氯丙烷相关蛋白1(Keap1)、核因子E2相关因子2(nuclear factor E2-related factor 2, Nrf2)和血红素加氧酶1(home oxygenase 1, HO-1)(北京鼎盛昌盛生物技术有限公司),苏木素伊红(HE)染色试剂盒(北京雷根生物技术有限公司),其他试剂均为国产分析纯。MT-200 Morris水迷宫分析仪、BA-200小鼠避暗仪和DT-200小鼠转棒测试仪(成都泰盟科技有限公司),Infinite M200型酶标仪(瑞士TECAN集团公司),低温高速离心机(美国Eppendorf公司),Western blotting凝胶电泳仪和转膜仪(美国Bio-Red公司),多色荧光及化学发光凝胶成像系统(北京赛智创业科技有限公司)。
1.2 实验动物分组和给药方式静坐实验:保持小鼠的正常生活,不施加任何外界干扰。游泳实验:采用游泳实验制备小鼠疲劳模型,将小鼠置于水深为20cm的恒温(20 ℃±2 ℃)水箱中进行游泳实验,第1天游泳30 min,第2天40 min,从第3天开始每天1 h,共4周。
将60只雄性ICR小鼠随机分为4组,每组15只。对照组:灌胃给予蒸馏水,静坐实验;模型组:灌胃给予蒸馏水,游泳实验;ELB(C)组:灌胃给予80 mg·kg-1 ELB,静坐实验;ELB(M)组:灌胃给予80 mg·kg-1 ELB,游泳实验。每天灌胃1次,共4周。
1.3 转棒实验末次给药30 min后,使DT-200小鼠转棒测试仪保持30 r·min-1的转速,小鼠先进行3次适应训练后进行正式实验,记录小鼠在转棒上运动的时间。
1.4 Morris水迷宫实验末次给药30 min后开始Morris水迷宫实验。实验前,将小鼠置于水迷宫装置中游泳2 d,保持每天2 min,使各组小鼠适应环境。定位航行测试:恒温水池(25℃±2 ℃,r=60 cm,h=50 cm)中放置1个低于水面1 cm的玻璃平台(r=2.5 cm),并加入适量二氧化钛(TiO2)。测试6 d,将小鼠面对水池壁放入恒温水池中游泳,仪器自动记录小鼠找到平台时间。若2 min未上台,则逃避潜伏期记为120 s。
1.5 避暗实验末次给药30 min后,先将小鼠置于BA-200小鼠避暗仪中充分适应环境,在暗室给予一个5 min的持续电流,当小鼠进入时将遭受电击,逃回明室。从实验开始到小鼠第一次遭受电击的时间即为避暗潜伏期,最长潜伏期为5 min。记录小鼠5 min之内遭受电击的总次数,即为避暗错误次数。
1.6 小鼠血清和脑组织中各生化指标检测末次给药30 min后,小鼠眼球取血,离心制备血清。采血后迅速取小鼠脑组织,称质量并用生理盐水漂洗,备用。采用比色法检测小鼠血清中LD水平,采用微量酶标法检测小鼠血清中LDH活性,脲酶法检测小鼠血清中BUN水平,羟胺法检测小鼠脑组织中SOD活性,硫代巴比妥酸(TBA)法检测小鼠脑组织中MDA水平,酶联免疫吸附测定法(ELISA)检测小鼠脑组织中ROS、eNOS、GABA和GLU水平,分光光度法检测小鼠脑组织中ATPase活性。血清中LDH活性(U·L-1)=[(测定A值-对照A值)/(标准A值-空白A值)]×标准品浓度×稀释倍数×1000,血清中LD和BUN水平(mmol·L-1)=[(测定A值-空白A值)/(标准A值-空白A值)]×标准品浓度×稀释倍数,ATPase活性(μmolPi·mg-1 prot·h-1)=[(测定A值-对照A值)/标准A值]×标准品浓度(0.5 mol·L-1) ×反应体系中样本稀释倍数(2.5)×6/匀浆蛋白浓度(g·L-1)。
1.7 Western blotting法检测小鼠脑组织中Keap1、Nrf2和HO-1蛋白表达水平末次给药30 min后,取小鼠脑组织在冰上裂解30 min,12 000 r·min-1离心5 min,取上清。根据BCA蛋白定量测定试剂盒具体步骤严格操作,测定小鼠脑组织的蛋白浓度。SDS-PAGE凝胶电泳分离各蛋白,冷水浴中电转膜70 min,转至聚偏二氟乙烯(PVDF)膜上,用含5 %脱脂奶粉的TBS-T封闭1 h,弃封闭液,分别加入一抗β-actin、Keap1、Nrf2和HO-1,4 ℃摇床过夜。TBS-T洗膜5次(15 min/次),加入二抗室温摇床孵育1 h,TBS-T洗膜5次(15 min/次),ECL显影液显色。目的蛋白表达水平=目的蛋白条带灰度值/β-actin条带灰度值。
1.8 HE染色观察小鼠脑组织病理形态表现末次给药30 min后,取小鼠全脑,投入4%多聚甲醛中固定,进行冰冻切片,HE染色按照HE染色试剂盒说明书进行操作。
1.9 统计学分析采用SPSS 19.0统计软件进行统计学分析。各组小鼠转棒时间、水迷宫潜伏期、避暗潜伏期和错误次数,小鼠血清和脑组织中各生化指标检测结果及GLU/GABA比值,小鼠脑组织中Keap1、Nrf2和HO-1蛋白表达水平,均符合正态分布,均以x±s表示,多组间样本均数比较采用单因素方差分析,组间样本均数两两比较采用SNK-q检验。以P<0.05为差异有统计学意义。
2 结果 2.1 各组小鼠转棒时间与对照组比较,模型组小鼠转棒时间明显缩短(P<0.05),ELB(C)组小鼠转棒时间延长(P<0.05);与模型组比较,ELB(M)组小鼠转棒时间明显延长(P<0.05),且各组小鼠第2天的转棒时间均较第1天延长。见表 1。
| (n=15, x±s, t/s) | |||||||||||||||||||||||||||||
| Group | Rotating rod time | ||||||||||||||||||||||||||||
| (t/d) 1 | 2 | ||||||||||||||||||||||||||||
| Control | 11.70±0.87 | 23.80±2.88 | |||||||||||||||||||||||||||
| Model | 6.20±0.54* | 16.40±1.61* | |||||||||||||||||||||||||||
| ELB(C) | 15.80±0.65* | 28.90±3.02* | |||||||||||||||||||||||||||
| ELB(M) | 10.30±0.39△ | 21.30±1.83△ | |||||||||||||||||||||||||||
| F | 255.686 | 189.316 | |||||||||||||||||||||||||||
| P | 0.01 | <0.01 | |||||||||||||||||||||||||||
| *P<0.05 vs control group;△P<0.05 vs model group. | |||||||||||||||||||||||||||||
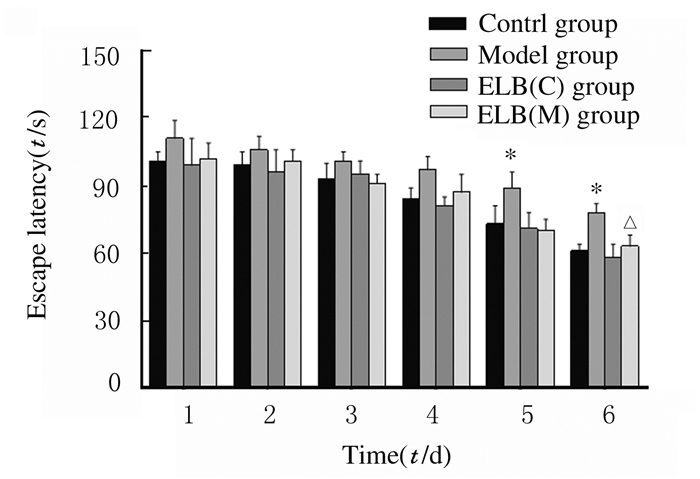
定位航行实验结果显示:各组小鼠找到平台的时间(逃避潜伏期)随着训练天数的增加而逐渐缩短。在实验第5和6天,与对照组比较,模型组小鼠逃避潜伏期明显延长(P<0.05),ELB(C)组小鼠逃避潜伏期缩短,但差异无统计学意义(P>0.05);与模型组比较,实验第6天ELB(M)组小鼠逃避潜伏期明显缩短(P<0.05)。见图 1。
|
| *P < 0.05 vs control group; △P < 0.05 vs model group. 图 1 各组小鼠水迷宫实验的逃避潜伏期 Fig. 1 Escape latencies of mice in various groupsin water mazetest |
|
|
各组小鼠避暗潜伏期随着时间的增加有不同程度的延长。与对照组比较,模型组小鼠避暗潜伏期缩短(P<0.05),ELB(C)组小鼠避暗潜伏期延长,但差异无统计学意义(P>0.05);与模型组比较,ELB(M)组小鼠避暗潜伏期明显延长(P<0.05)。各组小鼠避暗错误次数随着时间的延长均有不同程度的减少。与对照组比较,模型组小鼠5min内避暗错误次数明显增多(P<0.05),ELB(C)组小鼠避暗错误次数减少,但差异无统计学意义(P>0.05);与模型组比较,ELB(M)组小鼠避暗错误次数明显减少(P<0.05)。见表 2。
| (n=15, x±s) | |||||||||||||||||||||||||||||
| Group | Dark avoidance latency(t/s) | Number of dark avoidance errors | |||||||||||||||||||||||||||
| (t/d) 1 | 2 | 1 | 2 | ||||||||||||||||||||||||||
| Control | 25.90±5.35 | 57.40±3.63 | 4.80±0.32 | 4.20±0.41 | |||||||||||||||||||||||||
| Model | 14.20±3.28* | 29.80±5.32* | 6.60±0.37* | 5.80±0.33* | |||||||||||||||||||||||||
| ELB(C) | 27.60±4.51 | 61.50±4.89 | 4.40±0.42 | 3.7±0.64 | |||||||||||||||||||||||||
| ELB(M) | 22.50±2.08△ | 52.10±3.85△ | 5.30±0.26△ | 4.60±0.35△ | |||||||||||||||||||||||||
| F | 375.640 | 259.236 | 461.878 | 327.645 | |||||||||||||||||||||||||
| P | <0.01 | <0.01 | <0.01 | <0.01 | |||||||||||||||||||||||||
| *P<0.05 vs control group;△P<0.05 vs model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组小鼠血清中LDH活性和LD及BUN水平明显升高(P<0.05);ELB(C)组小鼠血清中LDH活性和LD及BUN水平降低,但差异无统计学意义(P>0.05)。与模型组比较,ELB(M)组小鼠血清中LDH活性和LD及BUN水平明显降低(P<0.05)。见表 3。
| (n=15, x±s) | |||||||||||||||||||||||||||||
| Group | LDH [λB/(U·L-1)] | LD [cB/(mmol·L-1)] | BUN [cB/(mmol·L-1)] | ||||||||||||||||||||||||||
| Control | 236.85±8.92 | 3.83±0.31 | 6.19±0.72 | ||||||||||||||||||||||||||
| Model | 382.53±14.73* | 6.47±0.54* | 8.25±0.49* | ||||||||||||||||||||||||||
| ELB(C) | 240.61±12.28 | 3.42±0.48 | 5.70±0.64 | ||||||||||||||||||||||||||
| ELB(M) | 285.06±9.13△ | 4.50±0.63△ | 5.91±0.37△ | ||||||||||||||||||||||||||
| F | 531.473 | 820.343 | 362.049 | ||||||||||||||||||||||||||
| P | <0.01 | <0.01 | <0.01 | ||||||||||||||||||||||||||
| *P<0.05 vs control group;△P<0.05 vs model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组小鼠脑组织中SOD活性、eNOS水平和GLU/GABA比值降低(P<0.05),MDA和ROS水平升高(P<0.05);ELB(C)组小鼠脑组织中SOD活性、eNOS水平和GLU/GABA比值升高,MDA和ROS水平降低,但差异无统计学意义(P>0.05)。与模型组比较,ELB(M)组小鼠脑组织中SOD活性、eNOS水平和GLU/GABA比值升高(P<0.05),MDA和ROS水平降低(P<0.05)。见表 4。
| (n=15, x±s) | |||||||||||||||||||||||||||||
| Group | SOD [λB/(U·mL-1)] |
MDA [cB/(mol·L-1)] |
ROS [λB/(U·mL-1)] |
eNOS [λB/(U·L-1)] |
GLU/GABA | ||||||||||||||||||||||||
| Control | 141.33±4.08 | 6.27±0.41 | 71.41±4.03 | 7.83±0.22 | 3.73±0.51 | ||||||||||||||||||||||||
| Model | 95.05±6.23* | 8.52±0.57* | 89.55±3.60* | 5.90±0.61* | 2.31±0.25* | ||||||||||||||||||||||||
| ELB(C) | 158.46±6.37 | 5.93±0.74 | 68.17±2.93 | 8.15±0.54 | 4.02±0.69 | ||||||||||||||||||||||||
| ELB(M) | 132.43±3.16△ | 6.37±0.52△ | 75.36±2.59△ | 7.60±0.48△ | 3.34±0.44△ | ||||||||||||||||||||||||
| F | 78.783 | 102.815 | 137.445 | 84.403 | 141.697 | ||||||||||||||||||||||||
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||||||||||||||||
| *P<0.05 vs control group;△P<0.05 vs model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组小鼠脑组织中Na+K+-ATPase、Mg2+-ATPase、Ca2+-ATPase和Ca2+Mg2+-ATPase活性降低(P<0.05);ELB(C)组小鼠脑组织中Na+K+-ATPase、Mg2+-ATPase、Ca2+-ATPase和Ca2+Mg2+-ATPase活性升高,但差异无统计学意义(P>0.05)。与模型组比较,ELB(M)组小鼠脑组织中Na+K+-ATPase、Mg2+-ATPase、Ca2+-ATPase和Ca2+Mg2+-ATPase活性升高(P<0.05)。见表 5。
| (n=15, x±s, μmol Pi·mg-1 prot·h-1) | |||||||||||||||||||||||||||||
| Group | Na+K+-ATPase | Mg2+-ATPase | Ca2+-ATPase | Ca2+Mg2+-ATPase | |||||||||||||||||||||||||
| Control | 67.04±7.35 | 89.36±5.72 | 110.74±12.03 | 152.87±15.35 | |||||||||||||||||||||||||
| Model | 52.08±5.17* | 63.07±6.31* | 62.52±4.13* | 96.28±13.07* | |||||||||||||||||||||||||
| ELB(C) | 76.63±10.02 | 93.42±8.74 | 114.09±9.61 | 178.81±10.93 | |||||||||||||||||||||||||
| ELB(M) | 61.75±6.43△ | 82.68±4.39△ | 121.30±6.75△ | 165.08±8.46△ | |||||||||||||||||||||||||
| F | 14.172 | 28.689 | 44.069 | 17.876 | |||||||||||||||||||||||||
| P | <0.01 | <0.01 | <0.01 | <0.01 | |||||||||||||||||||||||||
| *P<0.05 vs control group;△P<0.05 vs model group. | |||||||||||||||||||||||||||||
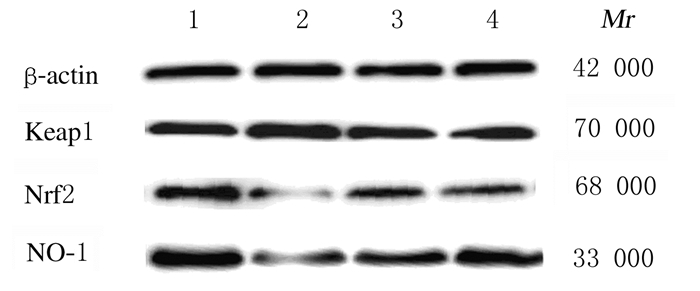
与对照组比较,模型组小鼠脑组织中Keap1蛋白表达水平升高(P<0.05),Nrf2和HO-1蛋白表达水平降低(P<0.05)。与模型组比较,ELB(M)组小鼠脑组织中Keap1蛋白表达水平降低(P<0.05),Nrf2和HO-1蛋白表达水平升高(P<0.05)。见图 2和表 6。
|
| Lane 1:Control group; Lane 2:Model group; Lane 3:ELB(C) group; Lane 4:ELB(M) group. 图 2 各组小鼠脑组织中Keap1、Nrf2和HO-1蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of Keap1, Nrf2, and HO-1 proteins in brain tissue of mice in various groups |
|
|
| (n=15, x±s) | |||||||||||||||||||||||||||||
| Group | Keap1/β-actin | Nrf2/β-actin | HO-1/β-actin | ||||||||||||||||||||||||||
| Control | 0.93±0.08 | 1.02±0.07 | 0.96±0.09 | ||||||||||||||||||||||||||
| Model | 1.42±0.10* | 0.47±0.04* | 0.42±0.09* | ||||||||||||||||||||||||||
| ELB(C) | 0.86±0.09 | 0.98±0.11 | 0.89±0.10 | ||||||||||||||||||||||||||
| ELB(M) | 0.91±0.09△ | 0.73±0.06△ | 0.82±0.08△ | ||||||||||||||||||||||||||
| F | 86.343 | 64.201 | 120.683 | ||||||||||||||||||||||||||
| P | <0.01 | <0.01 | <0.01 | ||||||||||||||||||||||||||
| *P<0.05 vs control group;△P<0.05 vs model group. | |||||||||||||||||||||||||||||
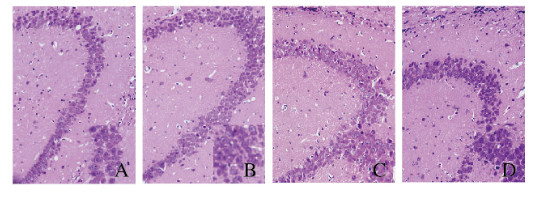
对照组小鼠脑组织海马区椎体细胞排列紧密有序,大小均匀,细胞核和细胞质清晰可见;模型组小鼠脑组织海马区神经元排列松散,层次不清,间隙增大,部分海马区神经元形态有明显的病理学改变;与对照组比较,ELB(C)组小鼠脑组织海马区神经元数量增多,层次清晰,排列较为紧密有序,细胞形态均一;与模型组比较,ELB(M)组小鼠脑组织海马区神经元排列更紧密有序,间隙减少,层次更加清晰,细胞形态和体积趋于正常。见图 3(插页六)。
|
| A: Control group; B: Model group; C: ELB (C) group; D: ELB (M) group. 图 3 各组小鼠脑组织海马区HE染色结果(×200) Fig. 3 Results of HE staining of hippocampus in brain tissue of mice in various groups(×200) |
|
|
超过耐受量的剧烈运动会产生明显的运动性疲劳,这时机体由有氧供能变成无氧糖酵解,使LD在体内积累,蛋白质分解加强,体内BUN水平升高,导致肌肉酸痛,使机体产生疲劳感同时运动耐力降低,而血清中LDH水平反映机体运动肌肉损伤的程度[10-11]。本研究通过游泳实验建立了运动性疲劳小鼠模型,采用转棒实验观察小鼠的运动耐力情况,结果显示:模型组小鼠转棒时间明显缩短,表明疲劳小鼠模型建立成功;与模型组比较,给予ELB干预后,疲劳小鼠血清中LD水平、LDH活性和BUN水平降低,提示ELB可加快LD的代谢,有效缓解疲劳。
疲劳可以引起机体神经系统机能和抗氧化功能改变,从而使学习记忆力降低[12-13]。疲劳时体内自由基不能得到有效清除,ROS积累在引起神经元损伤并导致认知功能障碍的同时,也造成eNOS脱偶联,NO水平下降和氧自由基水平升高,致使具有抗氧化作用的自由基清除酶SOD活性降低,反映自由基损伤的脂质过氧化产物MDA水平升高等[14-17]。本研究结果显示:疲劳模型小鼠水迷宫潜伏期延长,避暗潜伏期缩短、错误次数增加,小鼠脑组织中SOD活性和eNOS水平降低,MDA和ROS水平升高,说明疲劳小鼠处于氧化应激状态,且出现认知功能障碍;ELB干预后,疲劳小鼠水迷宫实验潜伏期缩短,避暗潜伏期延长、错误次数减少,小鼠脑组织中SOD活性和eNOS水平升高,MDA和ROS水平降低,说明ELB可有效增强疲劳小鼠抗氧化能力。疲劳小鼠体内ROS水平降低,则会使脑组织线粒体Ca2+内流减少,线粒体内ATPase的活性增高,中枢神经系统中与学习记忆相关的兴奋性神经递质GLU及抑制性神经递质GABA的比值升高[18-20],可降低脑内神经元损伤及受体功能障碍,改善疲劳小鼠的学习记忆能力。在本研究中,给予ELB干预后,疲劳小鼠脑组织中Na+K+-ATPase、Mg2+-ATPase、Ca2+-ATPase和Ca2+Mg2+-ATPase活性及GLU/GABA比值均升高,小鼠海马区结构层次清晰,排列较为紧密有序,提示ELB可以通过降低细胞氧化应激损伤,增强机体抗氧化作用,从而改善疲劳小鼠的学习记忆能力。
在细胞氧化应激反应中,Nrf2是关键因子,Keap1是Nrf2的负调节因子,正常情况下Keap1会与Nrf2在细胞浆结合,维持细胞质中Nrf2的稳定,当细胞处于氧化应激环境中时,Keap1与Nrf2解偶联,活化的Nrf2会进入细胞核与抗氧化反应元件ARE相互作用,调节抗氧化蛋白和编码细胞内解毒酶的表达,保护细胞[21-24]。而HO-1是Nrf2/ARE通路调控的重要抗氧化酶,受Nrf2调控。本研究结果显示:ELB干预后,疲劳小鼠脑组织中Keap1蛋白表达水平降低,Nrf2和HO-1蛋白表达水平升高,说明ELB改善疲劳小鼠的氧化应激状态与调控Keap1/Nrf2/ARE信号通路有关。
综上所述,ELB能够改善疲劳小鼠的学习记忆能力,增强机体抗氧化能力,具体机制可能与其调节Keap1/Nrf2/ARE信号通路的相关因子表达有关。本研究为刺五加的开发和利用奠定了理论和实践基础。
| [1] |
PENNER I K, PAUL F. Fatigue as a symptom or comorbidity of neurological diseases[J]. Nat Rev Neurol, 2017, 13(11): 662-675. DOI:10.1038/nrneurol.2017.117 |
| [2] |
YANG Q Y, LAI X D, OUYANG J, et al. Effects of Ginsenoside Rg3 on fatigue resistance and SIRT1 in aged rats[J]. Toxicology, 2018, 409: 144-151. DOI:10.1016/j.tox.2018.08.010 |
| [3] |
YU Q, WANG Y F, DU F, et al. Overexpression of endophilin A1 exacerbates synaptic alterations in a mouse model of Alzheimer's disease[J]. Nat Commun, 2018, 9(1): 2968. |
| [4] |
JIA N, SUN Q, SU Q, et al. Taurine promotes cognitive function in prenatally stressed juvenile rats via activating the Akt-CREB-PGC1α pathway[J]. Redox Biol, 2016, 10(1): 245-253. |
| [5] |
LAU K M, YUE G G, CHAN Y Y, et al. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components[J]. Chin Med, 2019, 14: 25. DOI:10.1186/s13020-019-0250-0 |
| [6] |
LIU S M, LI X Z, ZHANG S N, et al. Acanthopanax senticosus protects structure and function of mesencephalic mitochondria in a mouse model of Parkinson's disease[J]. Chin J Integrat Med, 2018, 24(11): 835-843. DOI:10.1007/s11655-018-2935-5 |
| [7] |
HUANG D B, HU Z H, YU Z F. Eleutheroside B or E enhances learning and memory in experimentally aged rats[J]. Neural Regen Res, 2013, 8(12): 1103-1112. |
| [8] |
LIU J J, ZHANG Z Z, GUO Q, et al. Syringin prevents bone loss in ovariectomized mice via TRAF6 mediated inhibition of NF-κB and stimulation of PI3K/AKT[J]. Phytomedicine, 2018, 42: 43-50. DOI:10.1016/j.phymed.2018.03.020 |
| [9] |
董杨. 刺五加苷B对MPP+损伤PC12细胞的神经保护作用[J]. 分子诊断与治疗杂志, 2014, 3(3): 155-158. |
| [10] |
ABBÈS S, BEN SALAH-ABBÈS J, JEBALI R, et al. Interaction of aflatoxin B1 and fumonisin B1 in mice causes immunotoxicity and oxidative stress:Possible protective role using lactic acid bacteria[J]. J Immunotoxicol, 2016, 13(1): 46-54. |
| [11] |
TOKUDOME Y. Influence of oral administration of lactic acid bacteria metabolites on skin barrier function and water content in a murine model of atopic dermatitis[J]. Nutrients, 2018, 10(12): E1858. DOI:10.3390/nu10121858 |
| [12] |
PROSCHINGER S, FREESE J. Neuroimmunological and neuroenergetic aspects in exercise-induced fatigue[J]. Exerc Immunol Rev, 2019, 25: 8-19. |
| [13] |
CHUNG Y H, PARK T K, YIM S H, et al. Polysaccharide-rich extract of Phragmites rhizome attenuates water immersion stress and forced swimming fatigue in rodent animal model[J]. J Med Food, 2019, 22(4): 355-364. |
| [14] |
ZHANG C, ZHANG L, LIU H, et al. Antioxidation, anti-hyperglycaemia and renoprotective effects of extracellular polysaccharides from Pleurotus eryngii SI-04[J]. Int J Biol Macromol, 2018, 111: 219-228. DOI:10.1016/j.ijbiomac.2018.01.009 |
| [15] |
ZHAO X, SONG J L, YI R K, et al. Comparison of antioxidative effects of insect tea and its raw tea (Kuding tea) polyphenols in Kunming mice[J]. Molecules, 2018, 23(1): E204. DOI:10.3390/molecules23010204 |
| [16] |
CHENG A J, BRUTON J D, LANNER J T, et al. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres[J]. J Physiol, 2015, 593(2): 163-172. |
| [17] |
WU H, WU Z G, SHI W J, et al. Effects of progesterone on glucose uptake in neurons of Alzheimer's disease animals and cell models[J]. Life Sci, 2019, 238: 116979. DOI:10.1016/j.lfs.2019.116979 |
| [18] |
张萍, 张良祥. 高压氧对运动疲劳小鼠肝脏组织抗氧化能力和ATP酶代谢的影响[J]. 吉林大学学报(医学版), 2018, 44(6): 1200-1204. |
| [19] |
LI N, LIU J L, WANG M Y, et al. Sedative and hypnotic effects of Schisandrin B through increasing GABA/Glu ratio and upregulating the expression of GABAA in mice and rats[J]. Biomed Pharmacother, 2018, 103: 509-516. DOI:10.1016/j.biopha.2018.04.017 |
| [20] |
ONODERA Y, TERAMURA T, TAKEHARA T, et al. MiR-155 induces ROS generation through downregulation of antioxidation-related genes in mesenchymal stem cells[J]. Aging Cell, 2017, 16(6): 1369-1380. DOI:10.1111/acel.12680 |
| [21] |
KAHROBA H, SHIRMOHAMADI M, HEJAZI M S, et al. The Role of Nrf2 signaling in cancer stem cells:From stemness and self-renewal to tumorigenesis and chemoresistance[J]. Life Sci, 2019, 239: 116986. DOI:10.1016/j.lfs.2019.116986 |
| [22] |
LIN G D, SUN Y W, LONG J H, et al. Involvement of the Nrf2-Keap1 signaling pathway in protection against thallium-induced oxidative stress and mitochondrial dysfunction in primary hippocampal neurons[J]. Toxicol Lett, 2020, 319: 66-73. DOI:10.1016/j.toxlet.2019.11.008 |
| [23] |
LIU Y W, LIU X L, KONG L, et al. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation[J]. Biomed Pharmacother, 2019, 109: 2145-2154. DOI:10.1016/j.biopha.2018.11.066 |
| [24] |
CHU S F, ZHANG Z, ZHOU X, et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway[J]. Acta Pharmacol Sin, 2019, 40(1): 13-25. |
 2020, Vol. 46
2020, Vol. 46


