扩展功能
文章信息
- 高瑞敏, 康玲玲, 侯防震
- GAO Ruimin, KANG Lingling, HOU Fangzhen
- 丹皮酚对心力衰竭大鼠心肌损伤的保护作用及其机制
- Protective effect of paeonol on myocardial injury in rats with heart failure and its mechanism
- 吉林大学学报(医学版), 2020, 46(04): 765-770
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 765-770
- 10.13481/j.1671-587x.20200416
-
文章历史
- 收稿日期: 2019-10-08
2. 河北医科大学附属唐山工人医院心内四科, 河北 唐山 063000
2. Fourth Department of Cardiology, Tangshan Worker's Hospital, Hebei Medical University, Tangshan 063000, China
慢性心力衰竭(chronic heart failure,CHF)是指由于心脏结构、心脏功能异常等导致心室充盈、射血能力受损引起的一种复杂的临床症状群,是绝大多数心脏疾病患者发展的终末阶段,病死率较高[1-2]。CHF多由心肌疾病、心肌梗死、血流动力学过度负荷和炎症爆发等原因导致心肌损伤,诱发心肌结构以及功能变化,使得心室泵血或充盈功能低下[3]。CHF发生时,心肌细胞持续凋亡,而剩余的心肌细胞功能逐渐退化诱发心脏功能不断恶化。因而心肌细胞的凋亡及心肌纤维化是心衰的主要病理基础[4]。近年来,越来越多的研究[5-6]显示:中药及其提取物在保护心脏、防止心肌细胞凋亡及纤维化等方面具有独特的作用。丹皮酚主要为中药丹皮或徐长卿等的活性成分,具有抗炎镇痛、神经保护、抑制脂质过氧化和抗过敏等作用,研究[7-8]显示:丹皮酚对缺血再灌注大鼠心肌具有保护作用。有研究者将培养的高血压病血瘀证患者的人脐静脉内皮细胞(human umbilical vein endothelial cells,HUVECs)分别用不同浓度的丹皮酚含药血清干预,证实丹皮酚能够很好调节患者免疫功能, 对高血压病患者血管内皮细胞有很好的保护作用[9]。但丹皮酚对CHF患者的心肌保护作用及其药理机制少见报道。本研究采用阿霉素尾静脉注射建立CHF大鼠模型,观察丹皮酚对CHF大鼠心功能的保护作用并探讨其药理学机制。
1 材料与方法 1.1 动物、主要试剂和仪器SPF级Wistar大鼠60只,雄性,体质量180~220g,购自河北省实验动物生产有限公司, 动物使用许可证号为SYXK(冀)2012-0002。实验操作严格按照我国《实验动物管理条例》进行,实验动物均饲养于SPF级环境中,不限供应干净水并按标准供应动物饲料,室内环境控制在日夜交替12h/12 h,温度控制在20℃~26℃,湿度控制在50% ~60%范围内。大鼠肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)和白细胞介素6 (interleukin-6,IL-6)ELISA试剂盒(美国Biolegend有限公司),鼠抗人Bax、B细胞淋巴瘤-2 (B-cell lymphoma-2,Bcl-2)、半胱氨酸天冬氨酸蛋白酶3(Cysteine aspartic acid protease-3, Caspase-3)、半胱氨酸天冬氨酸蛋白酶8 (Cysteine aspartic acid protease-8, Caspase-8)、半胱氨酸天冬氨酸蛋白酶9(Cysteine aspartic acid protease-9, Caspase-9)和细胞色素C (cytochrome C,Cyt-C)单克隆抗体和鼠抗人GADPH单克隆抗体(美国Santa Cruz公司),阿霉素注射剂(山西普德药业股份有限公司,批号151004),丹皮酚片剂(广西亿康药业股份有限公司,国药准字H45021119)。立式压力蒸汽灭菌器、恒温培养箱、电子天平、紫外可见分光光度计、光学显微镜和低温高速离心机均由本院中心实验室提供。
1.2 动物模型建立和分组给药大鼠适应性饲养1周后,参照文献[10]方法复制大鼠CHF模型。阿霉素总剂量15 mg·kg-1,每次2.5 mg·kg-1(10 mL·kg-1),1周3次腹腔内注射,共进行2周。成模标准:首次阿霉素注射5周后行超声心动图检查,大鼠心腔扩大且左室短轴缩短率(left ventricular fractional shortening,LVFS)<30%。成模大鼠共43只,随机分为4组:模型组(10只)、卡托普利组(11只)、低剂量丹皮酚组(11只)和高剂量丹皮酚组(11只)。卡托普利组大鼠灌胃给予卡托普利25 mg·kg-1,低和高剂量丹皮酚组大鼠分别灌胃给予75和150 mg·kg-1丹皮酚[11],每日1次,连续给药5周。另随机选取同批次正常大鼠10只作为对照组,对照组和模型组大鼠灌胃给予等量蒸馏水。每周记录体质量1次。
1.3 各组大鼠心功能指标检测各组大鼠腹腔注射2%戊巴比妥钠(50 mg·kg-1)麻醉,仰位固定,颈部正中切口,钝性分离右侧颈总动脉,结扎远心端,将充满1%肝素的导管自右颈总动脉徐缓进入,另一端连接PowerLab生物信号采集与分析系统,密切观察压力曲线,待导管进入左心室,即示波显示为左心室压力曲线后,稳定5 min,记录心功能指标。检测指标包括平均动脉压(mean arterial pressure, MAP)、心率(heart rate, HR)、左室舒张末压(left ventricular end diastolic pressure,LVEDP)、左室收缩压(1eft ventricular systolic pressure,LVSP)、左室内压最大下降速率(1eft ventricular maximal rates of pressure fall,-dp/dtmax)和左室内压最大上升速率(1eft ventricular maximal rates of pressure rise,+dp/dtmax)。连续测量5次,每次间隔3 min,以平均值作为结果。
1.4 ELISA法检测大鼠血清中TNF-α和IL-6水平末次给药后,麻醉大鼠,取眼眶外周血分离血清,采用ELISA试剂盒检测大鼠血清中血清中TNF-α和IL-6水平,单位为ng·L-1。
1.5 Western blotting法检测各组大鼠心肌组织中凋亡相关蛋白表达水平末次给药后,随机选取5只大鼠颈椎脱臼处死,在无菌操作下取出心脏组织于蛋白裂解缓冲液中匀浆, 按照参考文献[12-13]的方法,分离提取各个大鼠心脏总蛋白,蛋白于SDS-PAGE胶上进行电泳分离并转膜,脱脂牛奶(5%)封闭,于4 ℃摇床中用稀释1 000倍的一抗(Bax、Bcl-2、Caspase-3、Caspase-8、Caspase-9、Cyt-C和GADPH)孵育过夜,PBS洗膜3次,稀释5 000倍的二抗孵育1.5 h, PBS洗膜3次,ECL化学发光试剂盒显像。利用化学发光荧光成像仪的图像分析功能对条带灰度进行分析,以目的蛋白条带灰度值与内参条带灰度值的比值代表目的蛋白表达水平。
1.6 HE染色观察大鼠心肌组织病理形态表现末次给药后,随机选取5只大鼠麻醉后,在无菌操作下取出心脏,取一部分组织块置于10%甲醛溶液中固定,进行常规石蜡切片,厚度为4 μm,HE染色,中性树胶封片后于LEICA DMLB型光学显微镜下观察大鼠心脏组织病理形态表现。
1.7 统计学分析采用SPSS 19.0统计软件进行统计学分析。各组大鼠体质量、心功能指标、血清中TNF-α和IL-6水平及心肌组织中凋亡相关蛋白表达水平均符合正态分布, 均以x±s表示,多组间样本均数比较采用单因素方差分析,组间样本均数两两比较采用SNK-q检验。以P<0.05为差异有统计学意义。
2 结果 2.1 各组大鼠体质量造模前各组大鼠体质量比较差异无统计学意义(P>0.05);造模后,与对照组比较,模型组大鼠的体质量明显降低(P<0.05);给药后,与模型组比较,卡托普利组和低、高剂量丹皮酚组大鼠体质量明显增加(P<0.05),其中以高剂量丹皮酚组大鼠体质量增加更明显。见表 1。
| (x±s, m/g) | |||||||||||||||||||||||||||||
| Group | n | Body weight | |||||||||||||||||||||||||||
| Before modeling | After modeling | After last administration | |||||||||||||||||||||||||||
| Control | 10 | 193.3±7.2 | 210.1±8.1 | 212.1±6.5 | |||||||||||||||||||||||||
| Model | 10 | 196.2±3.9 | 156.2±3.7* | 135.2±10.2* | |||||||||||||||||||||||||
| Captopril | 11 | 193.8±5.6 | 152.4±6.3* | 162.1±8.3△ | |||||||||||||||||||||||||
| Low dose of paeonol | 11 | 191.4±4.2 | 154.6±4.9* | 168.4±7.9△ | |||||||||||||||||||||||||
| High dose of paeonol | 11 | 195.2±6.6 | 157.8±6.8* | 172.7±9.3△ | |||||||||||||||||||||||||
| *P<0.05 compared with control group;△P<0.05 compared with model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组大鼠HR降低(P<0.05),MAP明显升高(P<0.05);与模型组比较,卡托普利组和高剂量丹皮酚组大鼠HR升高(P<0.05),MAP明显降低(P<0.05);与卡托普利组比较,低和高剂量丹皮酚组大鼠HR和MAP差异无统计学意义(P>0.05)。与对照组比较,模型组大鼠LVSP、+dp/dtmax和-dp/dtmax明显降低(P<0.05),LVEDP明显升高(P<0.05);与模型组比较,卡托普利组及低、高剂量丹皮酚组大鼠LVSP、+dp/dtmax和-dp/dtmax均不同程度升高(P<0.05),LVEDP不同程度降低(P<0.05),其中以高剂量丹皮酚组效果较好。见表 2。
| (x±s) | |||||||||||||||||||||||||||||
| Group | n | HR (beat·min-1) |
MAP (P/mmHg) |
LVSP (P/mmHg) |
LVEDP (P/mmHg) |
+dp/dtmax (mmHg·s-1) |
-dp/dtmax (mmHg·s-1) |
||||||||||||||||||||||
| Control | 10 | 403.3±17.2 | 92.1±8.0 | 123.1±7.2 | 7.1±1.0 | 4 123.1±217.2 | 3 347.1±221.0 | ||||||||||||||||||||||
| Model | 10 | 386.2±13.0* | 113.2±6.9* | 86.2±3.0* | 13.2±2.6* | 2 786.2±193.0* | 2 313.2±252.6* | ||||||||||||||||||||||
| Captopril | 11 | 393.4±14.6△ | 101.8±3.3△ | 113.8±10.2△ | 9.9±1.5△ | 3 503.8±307.2△ | 2 979.3±361.3△ | ||||||||||||||||||||||
| Low dose of paeonol | 11 | 398.6±12.2 | 99.1±5.9 | 98.5±8.3△ | 10.4±2.1△ | 3 123.1±298.2△ | 2 689.8±271.8△ | ||||||||||||||||||||||
| High dose of paeonol | 11 | 401.3±17.1△ | 95.7±4.0△ | 111.6±7.1△ | 11.6±1.9△ | 3 842.8±343.8△ | 3 071.1±303.9△ | ||||||||||||||||||||||
| *P<0.05 compared with control group;△P<0.05 compared with model group. | |||||||||||||||||||||||||||||
给药5 d后, 与对照组比较,模型组大鼠血清中TNF-α和IL-6水平明显升高(P<0.05);与模型组比较,卡托普利组和低、高剂量丹皮酚组大鼠血清中TNF-α和IL-6水平明显降低(P<0.05)。见表 3。
| [x±s, ρB/(ng·L-1)] | |||||||||||||||||||||||||||||
| Group | n | IL-6 | TNF-α | ||||||||||||||||||||||||||
| Control | 10 | 43.36±7.11 | 52.01±8.60 | ||||||||||||||||||||||||||
| Model | 10 | 576.22±40.20* | 883.12±36.69* | ||||||||||||||||||||||||||
| Captopril | 11 | 233.84±37.62△ | 312.88±35.33△ | ||||||||||||||||||||||||||
| Low dose of paeonol | 11 | 424.14±62.49△ | 488.12±48.03△ | ||||||||||||||||||||||||||
| High dose of paeonol | 11 | 251.89±38.60△ | 379.18±21.85△ | ||||||||||||||||||||||||||
| *P<0.05 compared with control group;△P<0.05 compared with model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组大鼠心肌组织中Bax、Caspase-3、Caspase-8、Caspase-9和Cyt-C蛋白表达水平明显升高(P<0.05),Bcl-2蛋白表达水平明显降低(P<0.05)。与模型组比较,卡托普利及各剂量丹皮酚组大鼠心肌组织中Bax、Caspase-3、Caspase-8、Caspase-9和Cyt-C蛋白表达水平明显降低(P<0.05),Bcl-2蛋白表达水平明显升高(P<0.05)。见图 1和表 4。
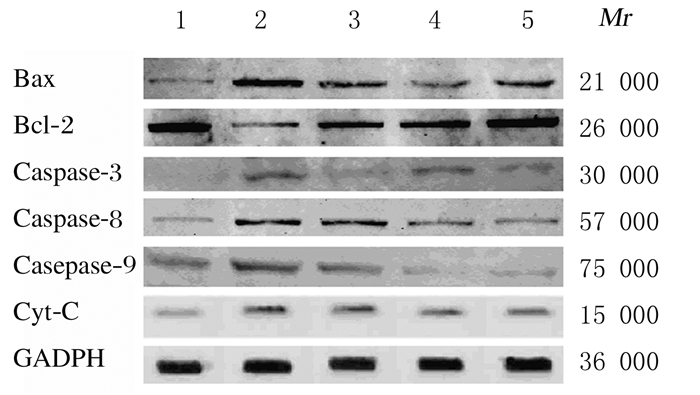
|
| Lane 1: Control group; Lane 2:Model group; Lane 3: High dose of paeonol group; Lane 4: Low dose of paeonol group; Lane 5: Captopril group. 图 1 各组大鼠心肌组织中凋亡相关蛋白表达电泳图 Fig. 1 Electrophoregram of expressions of apoptosis-related proteins in myocardium tissue of rats in various groups |
|
|
| (x±s) | |||||||||||||||||||||||||||||
| Group | n | Bax | Bcl-2 | Caspase-3 | Caspase-8 | Caspase-9 | Cyt-C | ||||||||||||||||||||||
| Control | 10 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||||||||
| Model | 10 | 1.42±0.13* | 0.48±0.06* | 1.23±0.14* | 1.28±0.15* | 1.19±0.16* | 1.44±0.18* | ||||||||||||||||||||||
| Captopril | 11 | 1.14±0.10△ | 1.13±0.11△ | 0.58±0.04△ | 1.03±0.09△ | 1.01±0.11△ | 1.23±0.14△ | ||||||||||||||||||||||
| Low dose of paeonol | 11 | 0.67±0.05△ | 1.05±0.09△ | 0.76±0.09△ | 1.02±0.07△ | 0.65±0.08△ | 1.15±0.11△ | ||||||||||||||||||||||
| High dose of paeonol | 11 | 0.97±0.08△ | 1.10±0.12△ | 0.68±0.05△ | 0.98±0.03△ | 0.70±0.10△ | 1.12±0.14△ | ||||||||||||||||||||||
| *P<0.05 compared with control group;△P<0.05 compared with model group. | |||||||||||||||||||||||||||||
对左心室进行横切,HE染色后观察:对照组大鼠心肌纤维排列整齐,未见破坏,细胞质丰富均匀,横纹规律,细胞间隙正常;模型组大鼠心肌组织受损明显,心肌细胞广泛水肿,并且出现空泡变性,受损严重的心肌细胞可见小灶状或片状坏死,部分心肌纤维断裂,肌原纤维溶解,心肌细胞间隙明显增宽;卡托普利组和低、高剂量丹皮酚组大鼠心肌组织可见轻度心肌细胞肥大,心肌纤维排列和心肌间质水肿等现象明显好转。见图 2(插页五)。

|
| A: Control group; B:Model group; C: High dose of paeonol group; D: Low dose of paeonol group; E: Captopril group. 图 2 各组大鼠心肌组织病理形态表现(HE,×400) Fig. 2 Pathomorphology of myocardium tissue of rats in various groups(HE, ×400) |
|
|
CHF发生发展的基本病理过程是心肌重构,其病理过程包括病理性心肌细胞肥大伴胚胎性基因再表达和心肌细胞凋亡与坏死、细胞外基质过度沉积和降解增加、心肌细胞分子结构的改变等[14],其中心肌细胞凋亡及心肌纤维化是CHF的主要病理基础。临床治疗中降低心肌细胞凋亡水平是CHF治疗的有效策略[15]。因为在CHF发生发展过程中,心肌细胞的凋亡可由氧化应激、容量或压力过度负荷及肾上腺素、血管紧张素Ⅱ和致炎细胞因子的过度释放以及缺血、缺氧等诱导发生。心肌细胞凋亡导致心肌细胞大量丢失,而剩余的心肌细胞常发生功能下降,当心肌细胞数量降低到一定程度后,会使得CHF进行性恶化,形成一个恶性循环的病理过程[16]。
本研究通过阿霉素腹腔注射构建CHF大鼠模型,结果显示:对照组大鼠外观和行为均正常,造模大鼠阿霉素注射5周后超声心动图检查显示心腔扩大,LVFS<30%,且造模大鼠毛发改变、皮肤紫绀、四肢水肿、呼吸频率加快和行动迟缓等均符合CHF的特征,证明成功建立CHF模型。HR、MAP、LVEDP、LVSP、+dp/dtmax和-dp/dtmax是动物实验评价心脏功能的关键指标[17-18]。本研究结果显示:模型组大鼠MAP、LVEDP升高而HR、LVSP、+dp/dtmax和-dp/dtmax明显降低,表明大鼠心脏功能明显下降,而经丹皮酚治疗后, 模型大鼠心功能指标明显改善,大鼠心肌组织中Bax、Caspase-3、Caspase-8、Caspase-9和Cyt-C凋亡相关蛋白表达水平明显降低,抗凋亡蛋白Bcl-2明显升高。本研究中组织形态学观察显示:丹皮酚能明显减轻CHF大鼠心肌损伤,改善心肌纤维断裂、心肌细胞水肿和结构破坏等,且高剂量丹皮酚组效果更好。有研究[19-20]表明:丹皮酚可增强抗氧化防御系统,激活Bax、Caspase-3、Caspase-8和Caspase-9等抗细胞凋亡信号通路因子,从而起到保护心肌的作用。临床研究[21-22]显示:CHF患者血清中炎症因子IL-6和TNF-α水平随着心功能的恶化而逐渐升高,IL-6和TNF-α水平是CHF严重程度的重要预测指标,细胞因子系统的持续激活是导致心室重塑和心衰恶化的重要原因。本研究选用经典的血管紧张素转换酶抑制剂卡托普利作为阳性对照药,结果显示:卡托普利对CHF大鼠体征及一般状态的改善效果较高剂量丹皮酚组差,对血清IL-6和TNF-α的抑制作用较不同剂量丹皮酚组作用强,提示卡托普利在抗炎和抗细胞损伤方面比较有优势,但是在心功能改善和抑制心肌纤维化方面,卡托普利与丹皮酚比较无明显差异,且高剂量丹皮酚在大鼠心功能保护及抗心肌细胞凋亡方面效果更好。
| [1] |
RONCO C, CICOIRA M, MCCULLOUGH P A. Cardiorenal syndrome type 1:pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure[J]. J Am Coll Cardiol, 2012, 60(12): 1031-1042. DOI:10.1016/j.jacc.2012.01.077 |
| [2] |
DAMMAN K, TESTANI J M. The kidney in heart failure:an update[J]. Eur Heart J, 2015, 36(23): 1437-1444. DOI:10.1093/eurheartj/ehv010 |
| [3] |
DAMMAN K, TANG W H, TESTANI J M, et al. Terminology and definition of changes renal function in heart failure[J]. Eur Heart J, 2014, 35(48): 3413-3416. DOI:10.1093/eurheartj/ehu320 |
| [4] |
BAGSHAW S M, CRUZ D N, ASPROMONTE N, et al. Epidemiology of cardio-renal syndromes:workgroup statements from the 7th ADQI Consensus Conference[J]. Nephrol Dial Transplant, 2010, 25(5): 1406-1416. DOI:10.1093/ndt/gfq066 |
| [5] |
叶婷, 张宇, 张梦, 等. 中医药治疗慢性心力衰竭药理机制研究进展[J]. 中西医结合心脑血管病杂志, 2016, 14(8): 841-843. DOI:10.3969/j.issn.1672-1349.2016.08.013 |
| [6] |
苏志远, 叶小汉, 吴锦波. 心康方治疗慢性心力衰竭临床观察[J]. 新中医, 2016, 48(3): 12-14. |
| [7] |
孙慧萍, 曹军平, 徐丽, 等. 丹皮酚对动脉粥样硬化大鼠炎症因子的影响[J]. 中华中医药学刊, 2016, 34(1): 14-16. |
| [8] |
LU L, QIN Y T, CHEN C, et al. Beneficial effects exerted by paeonol in the management of atherosclerosis[J]. Oxid Med Cell Longev, 2018, 2018: 1098617. |
| [9] |
张竞之, 陈小忆, 金伟孝, 等. 丹皮酚对高血压病血瘀证患者血清损伤的HUVEC-C形态学、活性的影响[J]. 辽宁中医药大学学报, 2012, 14(6): 29-31. |
| [10] |
陆建洪, 翟昌林, 陈捷. 丹皮酚对大鼠缺血再灌注损伤心肌细胞凋亡及其Bcl-2和Bax表达的影响[J]. 中国中医药科技, 2013, 20(2): 151-152. DOI:10.3969/j.issn.1005-7072.2013.02.033 |
| [11] |
SUEMATSU Y, JING W H, NUNES A, et al. LCZ696(sacubitril/valsartan), an angiotensin-receptor neprilysin inhibitor, attenuates cardiac hypertrophy, fibrosis, and vasculopathy in a rat model of chronic kidney disease[J]. J Card Fail, 2018, 24(4): 266-275. DOI:10.1016/j.cardfail.2017.12.010 |
| [12] |
HAO G P, ZHAI J, JIANG H M, et al. Acetylshikonin induces apoptosis of human leukemia cell line K562 by inducing S phase cell cycle arrest, modulating ROS accumulation, depleting Bcr-Abl and blocking NF-κB signaling[J]. Biomed Pharmacother, 2019, 122: 109677. |
| [13] |
LIU X Y, CHEN J Y, LI W, et al. Inhibition of casein kinase Ⅱ by CX-4945, but not yes-associated protein (YAP) by verteporfin, enhances the antitumor efficacy of temozolomide in glioblastoma[J]. Transl Oncol, 2020, 13(1): 70-78. |
| [14] |
BELOUSOV V V, FRADKOV A F, LUKYANOV K A, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide[J]. Nat Methods, 2006, 3(4): 281-286. DOI:10.1038/nmeth866 |
| [15] |
BERTERO E, MAACK C. Metabolic remodelling in heart failure[J]. Nat Rev Cardiol, 2018, 15(8): 457-470. DOI:10.1038/s41569-018-0044-6 |
| [16] |
VON LUEDER T G, WANG B H, KOMPA A R, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy[J]. Circ Heart Fail, 2015, 8(1): 71-78. |
| [17] |
BOEHM O, ZUR B, KOCH A, et al. Clinical chemistry reference database for Wistar rats and C57/BL6 mice[J]. Biol Chem, 2007, 388(5): 547-554. DOI:10.1515/BC.2007.061 |
| [18] |
BRAISSANT O, MCLIN V A, CUDALBU C. Ammonia toxicity to the brain[J]. J Inherited Metab Dis, 2013, 36: 595-612. DOI:10.1007/s10545-012-9546-2 |
| [19] |
ZHANG X X, ZHAI Y H, YUAN J H, et al. New insights into Paeoniaceae used as medicinal plants in China[J]. Sci Rep, 2019, 9(1): 18469. |
| [20] |
CHEN G, JIA P, YIN Z Y, et al. Paeonol ameliorates monosodium urate-induced arthritis in rats through inhibiting nuclear factor-κB-mediated proinflammatory cytokine production[J]. Phytother Res, 2019, 33: 2971-2978. DOI:10.1002/ptr.6472 |
| [21] |
BRAUNWALD E. Heart failure[J]. JACC, 2013, 1: 1-20. |
| [22] |
STEINHORN B, SORRENTINO A, BADOLE S, et al. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction[J]. Nat Commun, 2018, 9(1): 4044. |
 2020, Vol. 46
2020, Vol. 46


