扩展功能
文章信息
- 陈艳, 潘殿柱, 刘忠, 马艳梅, 张岚
- CHEN Yan, PAN Dianzhu, LIU Zhong, MA Yanmei, ZHANG Lan
- 沉默重组蛋白2对NCI-H1688细胞的抑制作用及其Wnt/β连环蛋白信号通路机制
- Inhibitory effect of sileneing Frat2 on NCI-H1688 cells and its Wnt/β catenin signaling pathway mechanism
- 吉林大学学报(医学版), 2020, 46(04): 751-758
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 751-758
- 10.13481/j.1671-587x.20200414
-
文章历史
- 收稿日期: 2019-10-15
T细胞淋巴瘤常见重排蛋白(frequently rearranged in advanced T-cell lymphomas,Frat)最早作为T细胞淋巴瘤原癌基因被发现,后来证实Frat为Wnt信号通路的重要组成部分。Frat包括Frat1和Frat2,研究[1-2]证实:Frat1通过Wnt信号通路在多种恶性肿瘤中发挥作用。张勇等[3]研究发现:Frat1在非小细胞肺癌组织中异常表达,其表达水平与肺癌细胞的侵袭和迁移等关系密切。Frat2也是Wnt信号通路的正向调节因子,但Frat2在肺癌中的作用尚不清楚。Wnt信号通路在小细胞肺癌细胞的增殖中发挥重要作用[4-5],而Frat2主要通过Wnt信号通路发挥作用,故本研究对NCI-H1688细胞中Frat2的表达及沉默Frat2对NCI-H1688细胞增殖及Wnt信号通路的影响进行研究,探讨Frat2对小细胞肺癌的可能作用机制。XAV939为Wnt/β连环蛋白(β-catenin)信号通路抑制剂,可抑制Wnt/β-catenin的调节转录,本研究同时对NCI-H1688细胞给予XAV939处理,进一步验证Frat2对小细胞肺癌的作用是否与Wnt/β-catenin信号通路有关。
1 材料与方法 1.1 细胞、主要试剂和仪器人小细胞肺癌NCI-H1688细胞和正常支气管上皮样细胞HBE细胞(中国科学院上海细胞库)。Frat2-siRNA慢病毒及阴性对照慢病毒(上海吉凯基因构建制备),逆转录(reverse transcription,RT)试剂盒、TRIzol试剂、聚合酶链反应(polymerase chain reaction,PCR)试剂盒和ECL化学发光试剂盒(美国BPB公司),Wnt信号通路抑制剂XAV939、胰蛋白酶、MTT试剂和DMEM培养基(美国Sigma公司),鼠抗人Frat2单克隆抗体(货号:SC-100437)、鼠抗人增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)单克隆抗体(货号:SC-103214)、鼠抗人c-myc单克隆抗体(货号:SC-100801)、鼠抗人细胞周期蛋白D1(Cyclin D1)单克隆抗体(货号:SC-100025)、鼠抗人β-catenin单克隆抗体(货号:SC-100183)和鼠抗人磷酸化糖原合成酶激酶3(phosphorylated glycogen synthase kinase 3,pGSK-3β)单克隆抗体(货号:SC-10427)(美国Invitrogen公司)。PCR扩增仪(美国Gibco公司),流式细胞仪和酶标仪(美国Bio-Rad公司)。
1.2 Western blotting法检测NCI-H1688细胞和HBE细胞中Frat2蛋白表达水平取生长良好的NCI-H1688细胞和HBE细胞,加入蛋白裂解液裂解后,12 000 r·min-1离心10 min,取上清液采用BCA法测定蛋白浓度,在SDS-PAGE凝胶中加入30 μg蛋白样品,电泳、转膜,加入封闭液封闭2h,加入一抗:兔抗人Frat2单克隆抗体(1:200)过夜孵育,加入含辣根过氧化物酶标记的二抗孵育1h,ECL发光。以β-actin为内参,实验重复7次,采用凝胶电泳成像仪采集图像,Quantity One软件分析蛋白条带灰度值。Frat2蛋白表达水平以Frat2条带灰度值/β-actin条带灰度值比值表示。
1.3 逆转录实时荧光定量聚合酶链反应(Real-Time RT-qPCR)法检测NCI-H1688细胞和HBE细胞中Frat2 mRNA表达水平取生长良好的NCI-H1688细胞和HBE细胞加入TRIzol裂解液充分裂解,加入氯仿震荡15 s、静止3 min,2 000 r·min-1离心10 min,吸取上层水相溶液、异丙醇沉淀,乙醇洗涤,提取总RNA,逆转录为cDNA,进行Real-Time RT-qPCR,以GAPDH为内参。反应条件:95℃预变性60 s,60℃变性30 s,60℃退火30 s,共42个循环。引物序列:Frat2,上游5′-GTGGCTTCTCACCGAATCCAG-3′,下游5′-AGTGACTGAGTCCGGTCCG-3′,扩增长度325 bp; GAPDH,上游5′-GAAGTGAAGGTCGGAGTCA-3′,下游5′-TTCACACCCATGACGAACAT-3′,扩增长度402 bp。每组实验重复7次,以2-ΔΔCt法计算Frat2 mRNA表达水平。
1.4 细胞分组和慢病毒转染将NCI-H1688细胞随机分为空白对照组、阴性对照组、Frat2-siRNA组和Frat2-siRNA+XAV组。空白对照组细胞不转染,阴性对照组细胞转染阴性对照慢病毒,Frat2-siRNA组细胞转染Frat2-siRNA慢病毒,Frat2-siRNA+XAV组细胞转染Frat2-siRNA慢病毒并同时加入4μmol·L-1的Wnt信号通路抑制剂XAV939处理[5]。具体步骤:将各组NCI-H1688细胞洗涤,以5×104个/孔接种至6孔板中,吸出培养液上清后加入适量病毒液,使感染复数(MOI)为20,培养12 h,显微镜下观察各组细胞生长情况,转染4 d后检测转染效率,转染效率高于90%时用于实验。
1.5 转染效果测定取上述稳定转染的各组NCI-H1688细胞,采用Western blotting法和Real-Time RT-qPCR法测定各组NCI-H1688细胞中Frat2蛋白和mRNA表达水平,具体步骤同“1.2”和“1.3”。
1.6 MTT法检测各组NCI-H1688细胞增殖活性将上述各组转染后细胞接种到96孔板中,每孔1×104个细胞,培养24、48和72 h时分别在每孔细胞中加入20 μL MTT溶液,继续培养4 h,吸取培养液上清液,加入150 μL二甲基亚砜,震荡反应10 min,用酶联免疫检测仪测定490 nm波长处吸光度(A)值,代表细胞增殖活性,每组实验重复7次。
1.7 流式细胞术检测各组不同细胞周期细胞百分率取上述转染后各组NCI-H1688细胞,2 000 r·min-1离心10 min,弃去上清液,加入PBS液,调整细胞浓度为5×105 mL-1,加入乙醇固定12 h,加入PBS液重悬细胞,加入碘化丙啶染色液孵育30 min,采用流式细胞仪检测各组不同细胞周期NCI-H1688细胞百分率,每组实验重复7次。
1.8 Western blotting法检测各组NCI-H1688细胞中PCNA、c-myc、Cyclin D1、β-catenin和pGSK-3β蛋白表达水平取各组转染后NCI-H1688细胞,采用Western blotting法测定各蛋白浓度,一抗分别为兔抗人PCNA单克隆抗体、兔抗人c-myc单克隆抗体、兔抗人Cyclin D1单克隆抗体、兔抗人β-catenin单克隆抗体和兔抗人pGSK-3β单克隆抗体。具体方法同“1.2”。
1.9 统计学分析采用SPSS20.0统计软件进行统计学分析。各组细胞中Frat2蛋白和mRNA表达水平、细胞增殖活性、不同细胞周期细胞百分率和细胞中PCNA、c-myc、Cyclin D1、β-catenin及pGSK-3β蛋白表达水平均符合正态分布,以x±s表示,2组间样本均数比较采用t检验,多组间样本均数比较采用单因素方差分析,组间两两比较采用LSD检验。以P<0.05为差异有统计学意义。
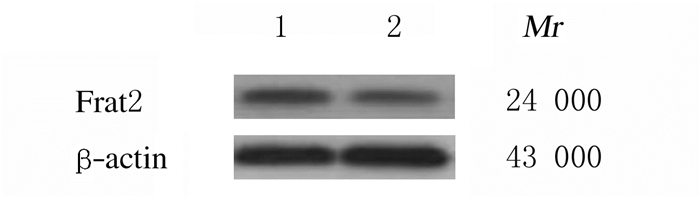
2 结果 2.1 NCI-H1688细胞和HBE细胞中Frat2蛋白和mRNA表达水平NCI-H1688细胞中Frat2蛋白和mRNA表达水平明显高于HBE细胞(P<0.01)。见表 1和图 1。
| (n=7, x±s) | |||||||||||||||||||||||||||||
| Cell | Frat2 protein | Frat2 mRNA | |||||||||||||||||||||||||||
| NCI-H1688 | 0.37±0.06 | 2.34±0.32 | |||||||||||||||||||||||||||
| HBE | 0.12±0.03 | 1.00±0.11 | |||||||||||||||||||||||||||
| t | 9.860 | 10.477 | |||||||||||||||||||||||||||
| P | <0.01 | <0.01 | |||||||||||||||||||||||||||
|
| Lane 1:NCI-H1688 cells; Lane 2:HBE cells. 图 1 Western blotting法检测NCI-H1688细胞和HBE细胞中Frat2蛋白表达电泳图 Fig. 1 Electrophoregram of expressions of Frat2 protein in NCI-H1688 and HBE cells detected by Western blotting method |
|
|
与空白对照组和阴性对照组比较,Frat2-siRNA组NCI-H1688细胞中Frat2蛋白和mRNA表达水平明显升高(P<0.05);与空白对照组比较,阴性对照组NCI-H1688细胞中Frat2蛋白和mRNA表达水平差异无统计学意义(P>0.05)。见表 2和图 2。
| (n=7, x±s) | |||||||||||||||||||||||||||||
| Group | Frat2 protein | Frat2 mRNA | |||||||||||||||||||||||||||
| Blank control | 0.43±0.08 | 1.00±0.11 | |||||||||||||||||||||||||||
| Negative control | 0.39±0.09 | 0.98±0.09 | |||||||||||||||||||||||||||
| Frat2-siRNA | 0.16±0.07*△ | 0.47±0.12*△ | |||||||||||||||||||||||||||
| F | 22.985 | 54.766 | |||||||||||||||||||||||||||
| P | <0.01 | <0.01 | |||||||||||||||||||||||||||
| *P<0.05 compared with blank control group;△P<0.05 compared with negative control group. | |||||||||||||||||||||||||||||

|
| Lane 1:Blank control group; Lane 2:Negative control group; Lane 3:Frat2-siRNA group. 图 2 Western blotting法检测各组NCI-H1688细胞中Frat2蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of Frat2 protein in NCI-H1688 cells in various groups detected by Western blotting method |
|
|
细胞培养24 h时,各组细胞增殖活性比较差异无统计学意义(P>0.05)。细胞培养48和72 h时,与空白对照组和阴性对照组比较,Frat2-siRNA组和Frat2-siRNA+XAV组细胞增殖活性降低(P<0.05);与Frat2-siRNA组比较,Frat2-siRNA+XAV组细胞增殖活性降低(P<0.05);与空白对照组比较, 阴性对照组细胞增殖活性差异无统计学意义(P>0.05)。见表 3。
| (n=7, x±s) | |||||||||||||||||||||||||||||
| Group | Proliferation activity | ||||||||||||||||||||||||||||
| (t/h) 24 | 48 | 72 | |||||||||||||||||||||||||||
| Blank control | 0.39±0.07 | 0.79±0.13 | 1.35±0.24 | ||||||||||||||||||||||||||
| Negative control | 0.38±0.06 | 0.76±0.14 | 1.29±0.23 | ||||||||||||||||||||||||||
| Frat2-siRNA | 0.33±0.08 | 0.52±0.12*△ | 0.81±0.18*△ | ||||||||||||||||||||||||||
| Frat2-siRNA+XAV | 0.30±0.06 | 0.36±0.09*△# | 0.63±0.16*△# | ||||||||||||||||||||||||||
| F | 2.724 | 19.849 | 20.938 | ||||||||||||||||||||||||||
| P | 0.067 | <0.01 | <0.01 | ||||||||||||||||||||||||||
| *P<0.05 compared with blank control group;△P<0.05 compared with negative control group;#P<0.05 compared with Frat2-siRNA group. | |||||||||||||||||||||||||||||
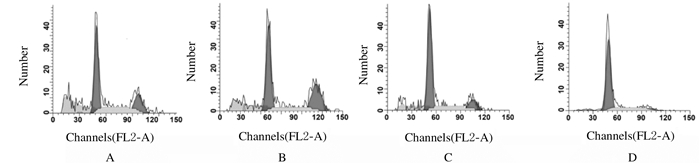
与空白对照组和阴性对照组比较,Frat2-siRNA组和Frat2-siRNA+XAV组细胞中G0 /G1期细胞百分率明显升高(P<0.05),S期和G2/M期细胞细胞百分率明显降低(P<0.05);与Frat2-siRNA组比较,Frat2-siRNA+XAV组细胞中G0/G1期细胞百分率升高(P<0.05),S期和G2/M期细胞百分率降低(P<0.05);与空白对照组比较,阴性对照组不同周期细胞百分率差异无统计学意义(P>0.05)。见表 4和图 3。
| (n=7, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | G0/G1 | S | G2/M | ||||||||||||||||||||||||||
| Blank control | 57.46±3.25 | 24.06±1.35 | 18.48±1.02 | ||||||||||||||||||||||||||
| Negative control | 58.38±3.61 | 23.12±1.42 | 18.50±0.96 | ||||||||||||||||||||||||||
| Frat2-siRNA | 66.59±3.72*△ | 19.83±1.52*△ | 13.58±0.87*△ | ||||||||||||||||||||||||||
| Frat2-siRNA+XAV | 78.73±3.81*△# | 16.27±1.39*△# | 5.00±0.93*△# | ||||||||||||||||||||||||||
| F | 52.350 | 43.275 | 316.290 | ||||||||||||||||||||||||||
| P | <0.01 | <0.01 | <0.01 | ||||||||||||||||||||||||||
| *P<0.05 compared with blank control group;△P<0.05 compared with negative control group;#P<0.05 compared with Frat2-siRNA group. | |||||||||||||||||||||||||||||
|
| A: Blank control group; B: Negative control group; C:Frat2-siRNA group; D:Frat2-siRNA+XAV group. 图 3 流式细胞术检测各组不同细胞周期NCI-H1688细胞百分率 Fig. 3 Percentages of NCI-H1688 cells in different cell cycles in various groups detected by flow cytometry |
|
|
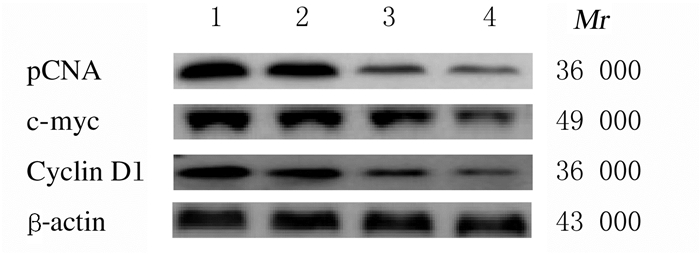
与空白对照组和阴性对照组比较,Frat2-siRNA组和Frat2-siRNA+XAV组细胞中PCNA、c-myc和Cyclin D1蛋白表达水平明显降低(P<0.05);与Frat2-siRNA组比较,Frat2-siRNA+XAV组细胞中PCNA、c-myc和Cyclin D1蛋白表达水平进一步降低(P<0.05);与空白对照组比较,阴性对照组细胞中PCNA、c-myc和Cyclin D1蛋白表达水平差异无统计学意义(P>0.05)。见表 5和图 4。
| (n=7, x±s) | |||||||||||||||||||||||||||||
| Group | PCNA | c-myc | Cyclin D1 | ||||||||||||||||||||||||||
| Blank control | 0.53±0.11 | 0.95±0.16 | 0.38±0.07 | ||||||||||||||||||||||||||
| Negative control | 0.49±0.12 | 0.93±0.14 | 0.37±0.08 | ||||||||||||||||||||||||||
| Frat2-siRNA | 0.22±0.06*△ | 0.52±0.15*△ | 0.25±0.06*△ | ||||||||||||||||||||||||||
| Frat2-siRNA+XAV | 0.10±0.05*△# | 0.21±0.11*△# | 0.11±0.05*△# | ||||||||||||||||||||||||||
| F | 37.362 | 44.313 | 25.680 | ||||||||||||||||||||||||||
| P | <0.01 | <0.01 | <0.01 | ||||||||||||||||||||||||||
| *P<0.05 compared with blank control group;△P<0.05 compared with negative control group;#P<0.05 compared with Frat2-siRNA group. | |||||||||||||||||||||||||||||
|
| Lane 1:Blank control group; Lane 2:Negative control group; Lane 3:Frat2-siRNA group; Lane 4:Frat2-siRNA+XAV group. 图 4 Western blotting法检测各组NCI-H1688细胞中PCNA、c-myc和Cyclin D1蛋白表达电泳图 Fig. 4 Electrophoresgram of expressions of PCNA, c-myc, Cyclin D1 proteins in NCI-H1688 cells in various groups detected by Western blotting method |
|
|
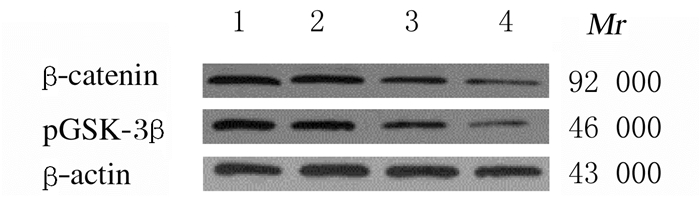
与空白对照组和阴性对照组比较,Frat2-siRNA组和Frat2-siRNA+XAV组细胞中β-catenin和pGSK-3β蛋白表达水平明显降低(P<0.05);与Frat2-siRNA组比较,Frat2-siRNA+XAV组细胞中β-catenin和pGSK-3β蛋白表达水平明显降低(P<0.05);与空白对照组比较,阴性对照组细胞中β-catenin和pGSK-3β蛋白表达水平差异无统计学意义(P>0.05)。见表 6和图 5。
| (n=7, x±s) | |||||||||||||||||||||||||||||
| Group | β-catenin | pGSK-3β | |||||||||||||||||||||||||||
| Blank control | 0.67±0.13 | 0.86±0.15 | |||||||||||||||||||||||||||
| Negative control | 0.66±0.14 | 0.85±0.16 | |||||||||||||||||||||||||||
| Frat2-siRNA | 0.32±0.11*△ | 0.35±0.10*△ | |||||||||||||||||||||||||||
| Frat2-siRNA+XAV | 0.15±0.09*△# | 0.14±0.09*△# | |||||||||||||||||||||||||||
| F | 32.823 | 55.577 | |||||||||||||||||||||||||||
| P | <0.01 | <0.01 | |||||||||||||||||||||||||||
| *P<0.05 compared with blank control group;△P<0.05 compared with negative control group;#P<0.05 compared with Frat2-siRNA group. | |||||||||||||||||||||||||||||
|
| Lane 1:Blank control group; Lane 2:Negative control group; Lane 3:Frat2-siRNA group; Lane 4:Frat2-siRNA+XAV group. 图 5 Western blotting法检测各组NCI-H1688细胞中β-catenin和pGSK-3β蛋白表达电泳图 Fig. 5 Electrophoregram of expressions of β-catenin and pGSK-3β proteins in NCI-H1688 cells in various groups detected by Western blotting method |
|
|
小细胞肺癌虽占全部肺癌的比例较少,但其发展快,恶性程度高,在早期可发生远处转移,大部分患者确诊时已有血行或淋巴转移,自然病程比较短;小细胞肺癌虽对放化疗比较敏感,但大部分患者治疗后可出现复发和转移[6]。因此探讨小细胞肺癌的发生发展机制,对小细胞肺癌的诊断及治疗具有重要意义。Frat位于第10号染色体长臂上,其主要功能是与GSK3结合,抑制GSK3对β-catenin的磷酸化,在wnt信号转导通路中起正向调节作用。Frat包括Frat1和Frat2,Frat1由279个氨基酸构成,相对分子质量为29 000;Frat2由233个氨基酸构成,相对分子质量为24 000。Frat1和Frat2有77%的氨基酸构成相同,与GSK-3β结合区的氨基酸全部相同;Frat1和Frat2均为Wnt信号通路的正向调节因子[7]。目前关于Frat1在恶性肿瘤中作用的研究较多,如MiR-34a-3p靶向Frat1可改变脑膜瘤细胞的增殖和凋亡[8];Frat1过表达可调节结肠癌细胞的增殖[9];沉默Frat1可抑制SGC7901人胃腺癌细胞增殖[10];敲低Frat1可通过抑制肝细胞癌细胞中的Wnt /β-catenin信号通路抑制缺氧诱导的上皮-间质转化[11];沉默Frat1基因可通过下调β-catenin表达影响结肠癌HT-29细胞增殖和凋亡[12];Frat1参与了前列腺癌的发生发展过程[13]。关于Frat2的研究较少,STOOTHOFF等[14]研究发现:Frat2与GSK-3β结合导致底物发生磷酸化从而发挥生物学效应。但Frat2在肺癌中的作用尚不清楚。本研究结果显示:沉默Frat2后NCI-H1688细胞中Frat2表达水平降低,NCI-H1688细胞增殖活性降低,表明Frat2可能参与小细胞肺癌的发生发展过程。
PCNA是DNA复制过程中所必需的蛋白,相对分子质量为36 000,在细胞核中合成,在G0 /G1期细胞中表达不明显,在G1晚期细胞中表达水平增加,S期细胞中表达水平达高峰,G2 /M期细胞中表达水平明显降低,与DNA的变化一致,是细胞增殖状态的标志物之一[15-16]。c-myc为myc家族成员,既是一种可易位基因,也是一种可调节基因,可使细胞获得永生化功能,使细胞无限增殖,促进细胞分裂,可决定细胞周期能否从G0 /G1期向S期过渡,是一种细胞恶性的标志物之一,与恶性肿瘤细胞的恶性增殖关系密切[17-18]。Cyclin D1为G1/S期特异性周期蛋白D1,属于高度保守的细胞周期家族成员之一,主要功能为促进细胞增殖,其作用在于作为细胞周期蛋白依赖性激酶的调控者,结合并激活G1期细胞特有的周期蛋白依赖性激酶CDK4,磷酸化G1期周期抑制蛋白,使其从所结合的E2F上解离,推动细胞周期从G1期进入S期[19-20]。本研究结果显示:沉默Frat2后NCI-H1688细胞中PCNA、c-myc和Cyclin D1蛋白表达水平降低,表明沉默Frat2可通过阻止G1期细胞向S期过渡进而抑制NCI-H1688细胞的增殖。
Wnt信号通路在恶性肿瘤细胞增殖中发挥重要作用,在肺癌的发生发展中也发挥重要作用。β-catenin为Wnt信号通路的关键调控因子,Wnt信号通路在正常成熟细胞中处于关闭状态,在病理状态下,Wnt信号通路被激活,β-catenin去磷酸化,进入细胞核与T细胞因子(T cell factor, TCF)/淋巴增强因子(lymphoid enhancer factor, LEF)结合开启下游的PCNA、c-myc和Cyclin D1等靶基因的转录,从而抑制恶性肿瘤细胞的增殖[21-22]。pGSK-3β是Wnt通路的关键调节因子,通过影响β-catenin参与调控细胞增殖和凋亡;经典Wnt通路激活后抑制由GSK-3β和轴蛋白等组成的复合体中的GSK-3β磷酸化,避免β-catenin被泛素蛋白酶体识别及降解,并与TCF/LEF结合导致细胞增殖异常,从而促进恶性肿瘤发生发展[23-24]。Wnt/β-catenin信号通路在肺癌细胞的增殖中发挥重要作用,且位于Frat2和Frat1与GSK-3β结合区的氨基酸全部相同,Frat2也可与GSK-3β结合发挥作用,进而调节Wnt信号通路,因此推测Frat2可能通过Wnt/β-catenin信号通路影响小细胞肺癌细胞的增殖。本研究结果显示:沉默Frat2后NCI-H1688细胞中β-catenin和pGSK-3β蛋白表达水平降低;对NCI-H1688细胞给予Wnt信号通路抑制剂XAV939后,NCI-H1688细胞的增殖能力降低,细胞被阻止在G0/G1期,细胞中PCNA、c-myc、Cyclin D1、β-catenin和pGSK-3β蛋白水平降低,表明沉默Frat2及给予Wnt信号通路抑制剂处理均可抑制小细胞肺癌细胞增殖、阻止细胞周期进程、抑制Wnt/β-catenin信号通路激活。由此可见Frat2可能通过Wnt/β-catenin信号通路抑制NCI-H1688细胞增殖。
综上所述,Frat2在小细胞肺癌NCI-H1688细胞中高表达,沉默Frat2可通过Wnt/β-catenin信号通路抑制NCI-H1688细胞增殖。Frat2有望成为小细胞肺癌治疗的潜在靶点。
| [1] |
刘景瑜, 曹欣, 郭庚, 等. FRAT1生物学特性的研究进展[J]. 国际遗传学杂志, 2015, 38(1): 35-40, 46. DOI:10.3760/cma.j.issn.1673-4386.2015.01.007 |
| [2] |
ZHANG B, LI N, ZHANG H. Knockdown of homeobox B5(HOXB5) inhibits cell proliferation, migration, and invasion in non-small cell lung cancer cells through inactivation of the Wnt/β-catenin pathway[J]. Oncol Res, 2018, 26(1): 37-44. |
| [3] |
张勇, 曹红一, 王恩华. Frat1与TCF4在非小细胞肺癌(NSCLC)中表达的相关性及意义[J]. 现代肿瘤医学, 2012, 20(6): 1175-1178. DOI:10.3969/j.issn.1672-4992.2012.06.24 |
| [4] |
PAN F, SHEN F Z, YANG L J, et al. Inhibitory effects of XAV939 on the proliferation of small-cell lung cancer H446 cells and Wnt/β-catenin signaling pathway in vitro[J]. Oncol Lett, 2018, 16(2): 1953-1958. |
| [5] |
李高兵, 蔡东平, 毛张凡. 川芎嗪通过调控Wnt信号通路抑制肺癌细胞增殖、侵袭和迁移的机制研究[J]. 现代肿瘤医学, 2019, 27(12): 2035-2040. DOI:10.3969/j.issn.1672-4992.2019.12.001 |
| [6] |
DIMAKAKOS E, LIVANIOS K, GKIOZOS I, et al. New data for venous thromboembolism in patients with small cell lung cancer:A review[J]. Phlebology, 2018, 33(8): 517-522. DOI:10.1177/0268355517737670 |
| [7] |
吕婷婷, 吕欣, 郭庚. FRAT1与肿瘤的相关性研究进展[J]. 医学综述, 2014, 20(20): 3691-3693. DOI:10.3969/j.issn.1006-2084.2014.20.017 |
| [8] |
WERNER T V, HART M, NICKELS R, et al. MiR-34a-3p alters proliferation and apoptosis of meningioma cells in vitro and is directly targeting SMAD4, FRAT1 and BCL2[J]. Aging (Albany NY), 2017, 9(3): 932-954. |
| [9] |
ZHU K X, GUO J Q, WANG H J, et al. FRAT1 expression regulates proliferation in colon cancer cells[J]. Oncol Lett, 2016, 12(6): 4761-4766. DOI:10.3892/ol.2016.5300 |
| [10] |
YU Q G, SHANG L, YU H B, et al. Silencing of FRAT1 by siRNA inhibits the proliferation of SGC7901 human gastric adenocarcinoma cells[J]. Biomed Rep, 2016, 4(2): 223-226. DOI:10.3892/br.2016.571 |
| [11] |
FAN W H, DU F J, LIU X J, et al. Knockdown of FRAT1 inhibits hypoxia-induced epithelial-to-mesenchymal transition via suppression of the Wnt/β-catenin pathway in hepatocellular carcinoma cells[J]. Oncol Rep, 2016, 36(5): 2999-3004. |
| [12] |
王苏雅, 于庆功, 谷伟, 等. 沉默FRAT1基因对结肠癌HT-29细胞增殖与凋亡的影响及其机制研究[J]. 中国实验诊断学, 2014, 18(2): 195-199. |
| [13] |
ZHANG W, XIONG H, ZOU Y M, et al. Frequently rearranged in advanced T cell lymphomas-1 demonstrates oncogenic properties in prostate cancer[J]. Mol Med Rep, 2016, 14(4): 3551-3558. |
| [14] |
STOOTHOFF W H, CHO J H, MCDONALD R P, et al. FRAT-2 preferentially increases glycogen synthase kinase 3 beta-mediated phosphorylation of primed sites, which results in enhanced tau phosphorylation[J]. J Biol Chem, 2005, 280(1): 270-276. |
| [15] |
卢迎宏, 王丹, 井海云. 苦参碱对oxLDL诱导的血管平滑肌细胞炎症反应及增殖凋亡的影响及分子机制研究[J]. 中国免疫学杂志, 2018, 34(4): 537-543. DOI:10.3969/j.issn.1000-484X.2018.04.012 |
| [16] |
JIA W, WU W, YANG D, et al. Histone demethylase JMJD3 regulates fibroblast-like synoviocyte-mediated proliferation and joint destruction in rheumatoid arthritis[J]. FASEB J, 2018, 32(7): 4031-4042. DOI:10.1096/fj.201701483R |
| [17] |
WANG Y, CONG W, WU G, et al. MiR-376a suppresses the proliferation and invasion of non-small-cell lung cancer by targeting c-Myc[J]. Cell Biol Int, 2018, 42(1): 25-33. |
| [18] |
ZOU J, LI X L, SHI Z M, et al. Effects of C-myc gene silencing on interleukin-1β-induced rat chondrocyte cell proliferation, apoptosis and cytokine expression[J]. J Bone Miner Metab, 2018, 36(3): 286-296. DOI:10.1007/s00774-017-0845-4 |
| [19] |
WU J H, ZHANG Y H, SUN L, et al. Cyclin D1 expression by histiocytes may mimic cyclin D1-positive proliferation centres of chronic lymphocytic leukaemia/small lymphocytic lymphoma[J]. Pathol Res Pract, 2018, 214(1): 72-75. |
| [20] |
CHEN J, LI X, CHENG Q, et al. Effects of cyclin D1 gene silencing on cellproliferation, cell cycle, and apoptosis of hepatocellular carcinoma cells[J]. J Cell Biochem, 2018, 119(2): 2368-2380. DOI:10.1002/jcb.26400 |
| [21] |
WANG B, SUN L W, LI J D, et al. MiR-577 suppresses cell proliferation and epithelial-mesenchymal transition by regulating the WNT2B mediated Wnt/β-catenin pathway in non-small cell lung cancer[J]. Mol Med Rep, 2018, 18(3): 2753-2761. |
| [22] |
WANG D P, GU L L, XUE Q, et al. CtBP2 promotes proliferation and reduces drug sensitivity in non-small cell lung cancer via the Wnt/β-catenin pathway[J]. Neoplasma, 2018, 65(6): 888-897. DOI:10.4149/neo_2018_171220N828 |
| [23] |
CHEN Z Y, DU Y, WANG L, et al. iR-543 promotes cell proliferation and metastasis of renal cell carcinoma by targeting Dickkopf 1 through the Wnt/β-catenin signaling pathway[J]. J Cancer, 2018, 9(20): 3660-3668. DOI:10.7150/jca.27124 |
| [24] |
GUO W X, SHEN F Z, XIAO W J, et al. Wnt inhibitor XAV939 suppresses the viability of small cell lung cancer NCI-H446 cells and induces apoptosis[J]. Oncol Lett, 2017, 14(6): 6585-6591. |
 2020, Vol. 46
2020, Vol. 46


