扩展功能
文章信息
- 杨帆, 李丽华
- YANG Fan, LI Lihua
- 维生素D受体激活对小鼠胆管结扎所致肝纤维化的影响及其机制
- Effect of vitamin D receptor activation on hepatic fibrosis induced by bile duct ligation in mice and its mechanism
- 吉林大学学报(医学版), 2020, 46(04): 722-727
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 722-727
- 10.13481/j.1671-587x.20200409
-
文章历史
- 收稿日期: 2019-11-05
肝纤维化是一种世界范围内的高发病率的肝脏疾病,威胁患者生命。肝纤维化具有可逆性特征,因此肝纤维化的研究已经成为近年来研究的热点。肝纤维化形成的中心环节是肝星状细胞(hepatic stellate cell,HSC)激活,激活的HSC释放细胞外基质(extracellular matrix,ECM),导致ECM在肝内累积,形成肝纤维化[1]。维生素D受体(vitamin D receptor,VDR)是核激素受体超家族的一员, 可被帕立骨化醇特异性激活。最近研究[2]表明: VDR可明显降低HSC促纤维化因子胶原蛋白Ⅰ(collagen Ⅰ,Col Ⅰ)的表达,缓解硫代乙酰胺导致的肝纤维化。VDR对胆总管结扎(bile duct ligation,BDL)导致的肝纤维化的作用机制尚不清楚。越来越多文献[3]证明:在氧化应激状态下,过量的氧自由基(reactive oxygen species,ROS)可以通过调节信号通路,激活HSC, 形成肝纤维化。VDR可以抑制线粒体膜电位,从而抑制ROS的产生,保护细胞免受氧化应激损伤[4]。此外,研究[5]显示:VDR激活过氧化物酶体增殖物激活受体-γ共激活因子1α(peroxisome proliferator-activated receptor γ coactivator-1,PGC-1α)使线粒体沉默交配型信息调节3(silence mating type information regulation 3,Sirt3)表达水平升高,降低骨骼肌中的氧化应激反应。Sirt3属于sirtuin家族,可调节线粒体蛋白的乙酰化并减少氧化应激[6]。Sirt3还参与通过调控琥珀酸脱氢酶(succinate dehydrogenase,SDH)活性抑制HSC的激活,减轻肝纤维化[7]。本文作者探讨激活的VDR通过调控Sirt3蛋白表达对BDL导致肝纤维化的影响,为其临床治疗提供新的理论依据。
1 材料与方法 1.1 实验动物、主要试剂和仪器SPF级C57BL/6小鼠60只,体质量22~25g,7~8周龄,购自北京维通利华实验动物技术有限公司, 动物生产许可证号:SCXK(京)2016-0006。小鼠在标准环境下饲养, 饮食自由。帕立骨化醇粉末(美国Sigma公司),HE染色试剂盒(北京索莱宝),二氢乙啶(dihydroethidium,DHE)染色试剂盒(上海碧云天生物技术有限公司),天狼猩红(Sirus red)试剂盒(北京索莱宝公司),Sirt3抗体和Col Ⅰ抗体(美国Abcam公司),8-羟基鸟嘌呤DNA糖苷酶1 (8-oxoguanine DNA glycosylase 1, OGG1)抗体、甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphate dehydrogenase,GADPH)抗体、α-平滑肌蛋白(alpha-smooth muscle,α-SMA)抗体和肌动蛋白β(beta actin,β-actin)抗体及HRP标记羊抗兔二抗和HRP标记羊抗鼠二抗均购买自美国Proteintech公司,ECL显影液(美国Bio-Rad公司)。ECL发光成像系统(美国Bio-Rad公司), Thermo Multisan MK3酶标仪(美国Thermo公司),Olympus BX-53型显微镜和Olympus DP-73倒置荧光显微镜均购自日本Olympus公司。
1.2 实验动物分组和药品配制60只C57BL/6小鼠随机分为假手术组、假手术后给药组、模型组和治疗组,每组15只。实验中动物饲养及使用均符合锦州医科大学动物伦理委员会要求。将帕立骨化醇粉末溶于60%的丙二醇溶液中,存储于-80℃冰箱。腹腔注射使用的溶液需要进一步用生理盐水稀释。根据有关文献[8]将帕立骨化醇的小鼠剂量确定为200 ng·kg-1。
1.3 BDL模型制备和用药小鼠适应性饲养1周进行实验。所有小鼠术前8h禁食,手术时室内温度保持在25℃左右。腹腔注射戊巴比妥钠(65 mg·kg-1)麻醉小鼠,乙醇消毒皮肤。模型组小鼠从剑突到耻骨联合进行腹部正中切口,暴露胆总管,双线结扎胆总管并中间离断,腹部缝合。假手术组和假手术后给药组小鼠除双线结扎胆管并中间离断外,其他手术过程均与模型组小鼠相同。假手术后给药组和治疗组小鼠在手术前3 d腹腔注射200 ng·kg-1帕立骨化醇溶液,每周3次,观察小鼠状态。手术结束后恢复性饲养,治疗组小鼠继续注射帕立骨化醇,于手术后的第5天取材。
1.4 HE染色观察小鼠肝组织病理形态表现用4%多聚甲醛固定小鼠肝组织,梯度酒精脱水、二甲苯透明、石蜡包埋后切片,石蜡烤片60℃过夜,二甲苯脱蜡梯度酒精复水后进行HE染色,显微镜下拍照,观察小鼠肝组织病理形态表现。依据《病毒性肝炎防治方案》[9], 将肝纤维化程度分为S0~S4期。S0期:汇管区无或轻微炎症,无纤维增生;S1期:汇管区轻微炎症、汇管区及中央静脉纤维化; S2期:汇管区炎症较轻,但其周围有纤维化并有纤维间隔形成;S3期:汇管区炎症较重、纤维间隔形成,无肝硬化;S4期:汇管区炎症严重, 假小叶形成。
1.5 天狼猩红染色观察小鼠肝组织纤维化程度冰冻切片恢复至室温,4%多聚甲醛固定组织, 用1×PBS洗涤3次, 每次5 min。天狼猩红染液染30 min。无水乙醇直接脱水,二甲苯透明后中性树胶封片。荧光显微镜下观察, 并采用图像分析系统进行分析。
1.6 DHE染色检测小鼠肝组织中ROS水平用4%多聚甲醛固定组织,30%蔗糖脱水,组织切片8μm,4%多聚甲醛固定组织15 min, 用1×PBS洗15 min,滴入DHE染液后37℃孵育30min;随后切片再用1×PBS洗涤3次, 每次5 min, 荧光显微镜下观察组织切片,并用Image J软件分析红色荧光强度,代表ROS水平。
1.7 Western blotting法检测小鼠肝组织中Sirt3、OGG1、α-SMA和Col Ⅰ蛋白表达水平取小鼠肝组织0.02 g,加入200μL蛋白裂解液,冰上充分匀浆裂解, 提取总蛋白液。BCA法进行蛋白定量,取约30μg蛋白进行10% SDS-PAGE电泳,90V恒压湿转90min,10% BSA封闭2 h。一抗(Sirt3、OGG1、α-SMA、Col Ⅰ均为1:1000稀释) 4℃孵育过夜,TBST洗涤3次,每次5min。室温下二抗孵育2h,TBST洗涤后ECL显影,暗室曝光。用Image J软件进行灰度值分析, 目的蛋白条带灰度值与内参照条带灰度值比值代表目的蛋白表达水平。
1.8 统计学分析采用SPSS17.0统计软件进行统计学分析。对各组小鼠肝组织中Sirt3、OGG1、α-SMA和Col Ⅰ蛋白表达水平进行正态分布和方差齐性检验, 为正态分布及方差齐, 均以x±s表示, 多组间样本均数比较采用单因素方差分析,组间两两比较采用LSD检验。检验水准为α=0.05。
2 结果 2.1 各组小鼠肝组织病理形态表现和纤维化程度HE染色结果显示:假手术组和假手术后给药组小鼠肝细胞排列整齐, 无炎性细胞浸润,无胆管增生;模型组小鼠肝组织炎性细胞浸润增多, 胆管明显增生;与模型组比较,治疗组小鼠肝组织炎性细胞浸润减少,肝组织坏死灶明显缩小(图 1,见插页二)。天狼猩红染色结果显示:假手术组和假手术后给药组小鼠肝组织无红色胶原纤维样物质围绕在胆管和大的静脉周围;与假手术组和假手术后给药组比较,模型组小鼠肝组织纤维化严重,在肝组织汇管区周围有大量红色胶原样纤维物质沉积;与模型组比较,治疗组小鼠肝组织汇管区及其大胆管周围红色纤维样物质明显减少,纤维化程度减轻(图 2,见插页二)。
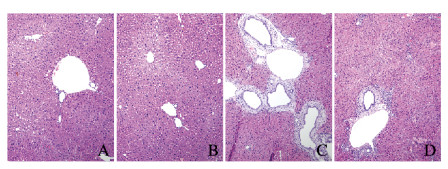
|
| A: Sham operation group; B: Sham operation + paricalcitol group; C:Model group; D:Treatment group. 图 1 各组小鼠肝组织病理形态表现(HE, ×100) Fig. 1 Pathomorphology of liver tissue of mice in various groups(HE, ×100) |
|
|
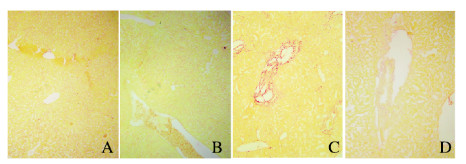
|
| A: Sham operation group; B: Sham operation + paricalcitol group; C: Model group; D: Treatment group. 图 2 天狼猩红染色观察各组小鼠肝组织纤维化情况(×100) Fig. 2 Fibrosis of liver tissue of mice in various groups observed by Sirus red staining (×100) |
|
|
依据《病毒性肝炎防治方案》[9],结合HE染色,将肝纤维化程度分为S0~S4期。假手术组和假手术后给药组S0期和S1期肝纤维化小鼠所占百分率总和高于模型组;模型组S4期肝纤维化小鼠所占百分率为53.3%,治疗组S4期肝纤维化小鼠所占百分率为6.6%,模型组S4期肝纤维化小鼠所占百分率远高于治疗组小鼠,肝纤维化严重。见表 1。
| Group | S0 stage | S1 stage | S2 stage | S3 stage | S4 stage |
| Sham operation | 15 | 0 | 0 | 0 | 0 |
| Sham operation+ paricalcitol | 14 | 1 | 0 | 0 | 0 |
| Model | 0 | 0 | 1 | 6 | 8 |
| Treatment | 0 | 4 | 7 | 3 | 1 |
DHE可以判断细胞中ROS水平[10]。假手术组和假手术后给药组小鼠汇管区及大胆管周围红色荧光弱;与假手术组和假手术后给药组比较,模型组小鼠肝组织大胆管周围及其汇管区红色荧光增强,ROS水平升高;与模型组比较,治疗组小鼠肝组织胆管周围红色荧光强度弱,ROS水平降低。见图 3(插页三)。
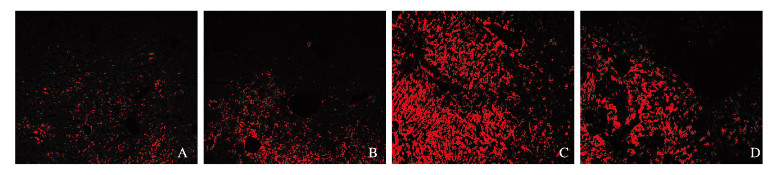
|
| A:Sham operation group; B:Sham operation+paricalcitol group; C:Model group; D:Treatment group. 图 3 各组小鼠肝组织DHE染色结果(×100) Fig. 3 DHE staining results of liver tissue of mice in various groups(×100) |
|
|
与假手术组和假手术后给药组比较,模型组小鼠肝组织中Sirt3蛋白表达水平明显降低(P<0.05), OGG1、α-SMA和Col Ⅰ蛋白表达水平明显升高(P<0.05);与模型组比较,治疗组小鼠肝组织中Sirt3蛋白表达水平明显升高(P<0.05),OGG1、α-SMA和Col Ⅰ蛋白表达水平明显降低(P<0.05)。见图 4和表 2。
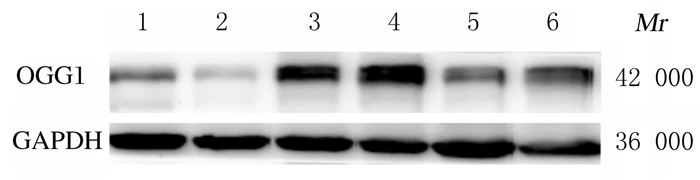
|
| Lane 1: Sham operation group; Lane 2: Sham operation+paricalcitol group; Lane 3, 4: Model group; Lane 5, 6: Treatment group. 图 4 各组小鼠肝组织中OGG1蛋白表达电泳图 Fig. 4 Electrophoregram of expression of OGG1 in liver tissue of mice in various groups |
|
|
| (n=15, x±s) | |||||||||||||||||||||||||||||
| Group | Sirt3 | OGG1 | α-SMA | Col Ⅰ | |||||||||||||||||||||||||
| Sham operation | 1.00±0.12 | 0.42±0.10 | 0.29±0.02 | 0.35±0.05 | |||||||||||||||||||||||||
| Sham operation+paricalcitol | 0.91±0.09 | 0.28±0.14 | 0.31±0.05 | 0.33±0.03 | |||||||||||||||||||||||||
| Model | 0.59±0.11*△ | 1.21±0.30*△ | 0.70±0.14*△ | 0.98±0.07*△ | |||||||||||||||||||||||||
| Treatment | 0.85±0.09# | 0.56±0.18# | 0.40±0.09# | 0.56±0.10# | |||||||||||||||||||||||||
| P | <0.05 | <0.01 | <0.05 | <0.05 | |||||||||||||||||||||||||
| *P<0.05 compared with sham operation group;△P<0.05 compared with sham operation+paricalcitol group;#P<0.05 compared with model group. | |||||||||||||||||||||||||||||
肝纤维化是一种发生于肝脏的炎症反应,多种疾病[11-12]均可导致肝纤维化。肝纤维化的过程涉及HSC的激活, α-SMA和Col Ⅰ是HSC激活的标志物[13-14]。正常的HSC处于静息状态,肝损伤时会激活HSC,HSC转化为肌成纤维细胞,后者释放大量ECM[1],ECM过度沉积,导致肝纤维化。胆汁淤积会导致肝细胞损伤,损伤持续性存在可激活HSC, 形成肝纤维化。VDR具有抗肝纤维化作用。DING等[15]研究发现:VDR可降低HSC中转化生长因子β(transforming growth factor-β, TGF-β)/Smad3基因的表达,减轻肝纤维化;DURAN等[16]研究表明:重组人死骨片1(recombinant human sequestosome 1, rhSQSTM1)通过上调VDR抑制小鼠肝脏纤维化。然而,关于VDR抑制BDL导致的肝纤维化机制尚不清楚。随着肝纤维化研究的深入,氧化应激与肝纤维化的关系逐渐受到重视。氧化应激造成过多ROS会刺激细胞引发脂质过氧化,使细胞的结构以及功能遭到破坏,造成肝细胞损伤及炎症反应,OGG1是氧化应激的标志蛋白[14],其表达水平变化可以反映机体的氧化应激情况。QUE等[17]发现:ROS会通过激活丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路,引发HSC的活化,导致肝纤维化。此外,VDR具有抗氧化应激的作用,如VDR抑制肝脏缺血再灌注小鼠氧化应激反应[18];抑制多肽脯氨酰基顺反异构酶介导的线粒体氧化应激来预防糖尿病内皮功能障碍[19];上调核因子E2相关因子2(nuclear factor E2 related factor 2, Nrf2),抑制氧化应激诱导的细胞衰老[20]。然而,VDR是否通过调控BDL小鼠的氧化应激缓解肝纤维化的情况尚不清楚。
VDR是核激素受体超家族的一员, 可与帕立骨化醇特异性结合[21]。VDR与维生素D的活性形式结合后,从细胞质进入细胞核,与维生素D受体反应原件(vitamin D receptor elements, VDREs)结合,调控靶基因[22]。在肝脏中,VDR表达于肝非实质细胞,如胆管上皮细胞、HSC和Kupffer细胞[23-25]。VDR在静息HSC中表达量很高,而当HSC被激活时VDR表达量可降低。向激活的HSC中加入1, 25 (OH)2-D3可刺激VDR的表达, 导致HSC趋于静息状态[2]。本研究结果显示:与模型组比较,治疗组小鼠肝组织中α-SMA和Col Ⅰ蛋白表达水平降低,表明激活的VDR可抑制HSC的激活。本研究中,HE染色和天狼猩红染色显示:与模型组比较,治疗组小鼠肝损伤和纤维化程度明显减轻;DHE染色结果显示:与模型组比较,治疗组小鼠肝组织DHE红色荧光的强度较强。DHE可透过细胞膜进入细胞内,并被细胞内的ROS氧化,形成氧化乙啶;氧化乙啶可掺入染色体DNA中,产生红色荧光[10],根据红色荧光的强度可以判断细胞ROS水平。本研究结果表明:氧化应激和肝纤维化有密切关联,激活VDR可抑制氧化应激导致的肝纤维化。
Sirt3是一种烟酰胺腺嘌呤二核苷酸(nicotinamide-adenine dinucleotide,NAD)依赖的Ⅲ型组蛋白去乙酰化酶(histone deacetylase,HDAC), 主要定位于细胞线粒体[26]。研究[5]显示:VDR上调线粒体Sirt3蛋白的表达,抑制骨骼肌氧化应激反应。CHENARI等[27]研究发现:Sirt3抑制氧化应激反应,缓解肝硬化。但尚无文献证明激活的VDR可以通过上调Sirt3蛋白抑制氧化应激。研究[28-30]表明:Sirt3能直接与超氧化物歧化酶2(superoxide dismutase 2, SOD2)的乙酰化位点结合, 使SOD2去乙酰化, 增加SOD2活性, 形成重要的Sirt3-SOD2抗氧化通路。此外,Sirt3刺激异柠檬酸转化为α-酮戊二酸, 使NAD磷酸化水平和线粒体中还原氧化型谷胱甘肽的比例增加, 保护细胞免受氧化应激的损伤[31]。本实验结果显示:Sirt3蛋白表达水平,BDL可导致小鼠肝组织中Sirt3蛋白表达水平降低,激活的VDR可以调Sirt3蛋白表达水平,同时降低OGG1表达,表明激活的VDR可抑制氧化应激反应,其机制是上调Sirt3蛋白。
本研究结果表明:在BDL小鼠模型中,激活的VDR通过上调Sirt3蛋白表达进而抑制氧化应激诱导的肝纤维化。因此,VDR激活将为肝纤维化的治疗提供一种新的思路。
| [1] |
ZHOU W C, ZHANG Q B, QIAO L. Pathogenesis of liver cirrhosis[J]. World J Gastroenterol, 2014, 20(23): 7312-7324. DOI:10.3748/wjg.v20.i23.7312 |
| [2] |
ABRAMOVITCH S, DAHAN-BACHAR L, SHARVIT E, et al. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats[J]. Gut, 2011, 60(12): 1728-1737. DOI:10.1136/gut.2010.234666 |
| [3] |
NGUYEN L A, HORUZSKO A. Kupffer cell metabolism and function[J]. Enzymol Metab, 2015, 1(1): 101. |
| [4] |
CONSIGLIO M, DESTEFANIS M, MORENA D, et al. The vitamin D receptor inhibits the respiratory chain, contributing to the metabolic switch that is essential for cancer cell proliferation[J]. PLoS One, 2014, 9(12): e115816. DOI:10.1371/journal.pone.0115816 |
| [5] |
呼德尔朝鲁.外源性维生素D3缓解运动诱发的慢性疲劳综合症及其骨骼肌抗氧化系统的影响与分子机制探讨[D].西安: 陕西师范大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10718-1014400002.htm
|
| [6] |
BROWN K, XIE S, QIU X L, et al. SIRT3 reverses aging-associated degeneration[J]. Cell Rep, 2013, 3(2): 319-327. |
| [7] |
LI Y H, CHOI D H, LEE E H, et al. Sirtuin 3(SIRT3) regulates α-smooth muscle actin (α-SMA) production through the succinate dehydrogenase-G protein-coupled receptor 91(GPR91) pathway in hepatic stellate cells[J]. J Biol Chem, 2016, 291(19): 10277-10292. DOI:10.1074/jbc.M115.692244 |
| [8] |
FAN Y G, GUO T, HAN X R, et al. Paricalcitol accelerates BACE1 lysosomal degradation and inhibits calpain-1 dependent neuronal loss in APP/PS1 transgenic mice[J]. EBio Medicine, 2019, 45: 393-407. |
| [9] |
徐列明, 刘平, 沈锡中, 等. 肝纤维化中西医结合诊疗指南(2019年版)[J]. 中国中西医结合杂志, 2019, 39(11): 1286-1295. DOI:10.7661/j.cjim.20190916.314 |
| [10] |
LI R, LIU Y, XIE J, et al. Sirt3 mediates the protective effect of hydrogen in inhibiting ROS-induced retinal senescence[J]. Free Rad Biol Med, 2019, 135: 116-124. DOI:10.1016/j.freeradbiomed.2019.02.005 |
| [11] |
WILHELM A, ALDRIDGEL V, HALDAR D, et al. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism[J]. Gut, 2016, 65(7): 1175-1185. DOI:10.1136/gutjnl-2014-308325 |
| [12] |
LIA D, REYES A, MELO CAMPOS, et al. Mitochondrial maintenance under oxidative stress depends on mitochondrially localised α-OGG1[J]. J Cell Sci, 2018. DOI:10.1242/jcs,213538 |
| [13] |
ZHANG C Y, YUAN W G, HE P, et al. Liver fibrosis and hepatic stellate cells:etiology, pathological hallmarks and therapeutic targets[J]. World J Gastroenterol, 2016, 22(48): 10512-10522. DOI:10.3748/wjg.v22.i48.10512 |
| [14] |
BENEDICT M, ZHANG X. Non-alcoholic fatty liver disease:An expanded review[J]. J Hepatol, 2017, 9(16): 715-732. |
| [15] |
DING N, YU R T, SUBRAMANIAM N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response[J]. Cell, 2013, 153(3): 601-613. |
| [16] |
DURAN A, HERNANDEZ E D, REINA-CAMPOS M, et al. P62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer[J]. Cancer Cell, 2016, 30(4): 595-609. |
| [17] |
QUE R, SHEN Y, REN J, et al. Estrogen receptor β dependent effects of saikosaponin D on the suppression of oxidative stress induced rat hepatic stellate cell activation[J]. Int J Mol Med, 2017, 41(3): 1357-1364. |
| [18] |
YAO T B, YING X Y, ZHAO Y C, et al. Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy[J]. Antioxid Redox Signal, 2015, 22(8): 633-650. DOI:10.1089/ars.2014.5887 |
| [19] |
ZHANG M J, LIN L M, XU C S, et al. VDR agonist prevents diabetic endothelial dysfunction through inhibition of prolyl isomerase-1-mediated mitochondrial oxidative stress and inflammation[J]. Oxid Med Cell Longev, 2018, 2018: 1714896. |
| [20] |
CHEN L L, YANG R L, QIAO W X, et al. 1, 25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling[J]. Aging Cell, 2019, 18(3): e12951. |
| [21] |
LEE S M, MEYER M B, BENKUSKY N A, et al. The impact of VDR expression and regulation in vivo[J]. J Steroid Biochem Mol Biol, 2018, 177: 36-45. DOI:10.1016/j.jsbmb.2017.06.002 |
| [22] |
LA MARRA F, STINCO G, BULIGAN C, et al. Immunohistochemical evaluation of vitamin D receptor (VDR) expression in cutaneous melanoma tissues and four VDR gene polymorphisms[J]. Cancer Biol Med, 2017, 14(2): 162-175. |
| [23] |
FIRRINCIELI D, ZUÑIGA S, REY C, et al. Vitamin D nuclear receptor deficiency promotes cholestatic liver injury by disruption of biliary epithelial cell junctions in mice[J]. Hepatology, 2013, 58(4): 1401-1412. |
| [24] |
KEANE J T, ELANGOVAN H, STOKES R A, et al. Vitamin D and the liver-correlation or cause?[J]. Nutrients, 2018, 10(4): 496. DOI:10.3390/nu10040496 |
| [25] |
刘智勇, 朱泽民. 肝脏巨噬细胞中维生素D受体的激活可以阻止小鼠肝脏内质网的应激[J]. 临床肝胆病杂志, 2019, 35(10): 2364. |
| [26] |
李霞, 王彦方, 陈战巧, 等. 沉默信息调节因子3在丹酚酸A抗紫外线诱导视网膜色素上皮细胞氧化应激性损伤中的作用及机制[J]. 温州医科大学学报, 2019, 49(4): 272-276. DOI:10.3969/j.issn.2095-9400.2019.04.008 |
| [27] |
CHENARI S, SAFARI F, MORADI A. Curcumin enhances liver SIRT3 expression in the rat model of cirrhosis[J]. Iran J Basic Med Sci, 2017, 20(12): 1306-1311. |
| [28] |
CHENG Y H, DAI C, ZHANG J. SIRT3-SOD2-ROS pathway is involved in linalool-induced glioma cell apoptotic death[J]. Acta Biochim Pol, 2017, 64(2): 343-350. |
| [29] |
CHEN Y, QING W, SUN M, et al. Melatonin protects hepatocytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway[J]. Free Radic Res, 2015, 49(10): 1275-1284. DOI:10.3109/10715762.2015.1067806 |
| [30] |
LIU X H, ZHANG L, WANG P, et al. Sirt3-dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice[J]. Cell Cycle, 2017, 16(13): 1302-1308. |
| [31] |
YU W, DITTENHAFER-REED K E, DENU J M. SIRT3 protein deacetylates isocitrate dehydrogenase 2(IDH2) and regulates mitochondrial redox status[J]. J Biol Chem, 2012, 287(17): 14078-14086. DOI:10.1074/jbc.M112.355206 |
 2020, Vol. 46
2020, Vol. 46


