扩展功能
文章信息
- 于雷, 王策, 韩冰, 李鑫, 韩雨辰, 孙宇莹, 郭湘舒, 刘威武, 王志成
- YU Lei, WANG Ce, HAN Bing, LI Xin, HAN Yuchen, SUN Yuying, GUO Xiangshu, LIU Weiwu, WANG Zhicheng
- 线粒体靶向KillerRed增强辐射诱导HeLa细胞自噬作用及其机制
- Enhancement of mitochondria-targeted KillerRed in autophagy caused by radiation in HeLa cells and its mechanism
- 吉林大学学报(医学版), 2020, 46(04): 693-698
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 693-698
- 10.13481/j.1671-587x.20200405
-
文章历史
- 收稿日期: 2019-10-22
2. 吉林大学第二医院放疗科, 吉林 长春 130041;
3. 吉林大学第二医院放射线科, 吉林 长春 130041;
4. 吉林省肿瘤医院放疗医技科, 吉林 长春 130012
2. Department of Radiotherapy, Second Hospital, Jilin University, Chanchun 130041, China;
3. Department of Radiology, Second Hospital, Jilin University, Chanchun 130041, China;
4. Department of Radiotherapy and Medical Technology, Jilin Cancer Hospital, Changchun 130012, China
活性氧(reactive oxygen species, ROS)是一类由线粒体产生的活性分子,作用于线粒体后可以导致线粒体膜电位(mitochondrial membrance potential, MMP)的降低和线粒体呼吸链的损伤,进而引起线粒体失能,并诱导细胞自噬[1-3]。本课题组前期研究[4-5]显示:线粒体靶向KillerRed(mitochondrion-targeted KillerRed, mtKR)可以增强辐射所致的细胞增殖抑制和凋亡,但其具体机制尚未明确。在本研究中,以构建的mtKR质粒Pink1-mtKR转染宫颈癌HeLa细胞,采用可见光和4 Gy X射线联合照射后,检测细胞MMP和氧化呼吸链复合物Ⅰ和Ⅲ的活性等功能指标,并检测自噬和PTEN诱导假定激酶1/帕金蛋白(PTEN-inducedputativekinase1/parkin,Pink1/parkin)调控通路相关蛋白表达,阐明mtKR对辐射诱导的线粒体失能和细胞自噬的作用,探讨其相关的分子调控机制,为增强宫颈癌放疗效果提供新思路。
1 材料与方法 1.1 细胞、主要试剂和仪器人宫颈癌HeLa细胞由本实验室保存。MEM完全培养基(美国Gibico公司),Hieff TransTM脂质体核酸转染试剂(上海翊圣生物科技有限公司),青、链霉素(美国Thermo Fisher Scientific公司),罗丹明123、线粒体呼吸链复合体Ⅰ和Ⅲ水平检测试剂盒(北京索莱宝科技有限公司),单丹磺酰尸胺(monodansylcadaverin, MDC,美国Sigma公司),线粒体分离试剂盒(碧云天生物技术有限公司),Pink1、parkin和线粒体外膜转位酶20(transcocase of outer mitochondrial membrance 20, Tom20)多克隆一抗(美国ImmunoWay公司),P62多克隆一抗(美国CST公司),热休克蛋白60(heat shock protein 60,Hsp60)和GAPDH兔多克隆一抗(美国Bioworld公司),辣根过氧化物酶标记的二抗(美国Santa Cruz公司),其他试剂均为国产分析纯。多功能微孔板检测仪(美国Biotek公司),X射线辐照仪(型号X-RAD 320iX,美国Precision X-ray股份有限公司)。
1.2 实验分组和照射HeLa细胞分为对照组、空载体组、Pink1-mtKR组、对照+4 Gy X射线照射组、空载体+4 GyX射线照射组和Pink1-mtKR+4 Gy X射线照射组。无菌条件下避光可见光源照射细胞[5],时间为30 min,12 h后X射线照射,条件为电压180 kV,电流12.0 mA,靶皮距70 cm,剂量率1.0 Gy·min-1,单次照射剂量为4 Gy。
1.3 流式细胞术检测细胞MMP和自噬率取对数生长期的HeLa细胞接种于24孔板中,细胞密度为0.7×105个/孔,瞬时转染空载体和Pink-mtKR质粒,30 h后给予30 min可见光照射,12 h后进行4 Gy X射线照射,于24 h时收集各组细胞,PBS洗涤3次。检测MMP时,加入300 μL PBS,重悬细胞,加入罗丹明123(终浓度5 μmol·L-1)于37℃避光反应30min,上机检测,以平均荧光强度(mean fluorescence intensity, MFI)表示MMP。加入10 mL MDC (5 mmol·L-1),37℃静置30 min后,1 000 r·min-1离心5 min,弃废液,加入4%多聚甲醛1 mL避光条件下固定15 min,离心后加入PBS洗涤1次,上机检测细胞自噬率。
1.4 细胞中线粒体呼吸链复合物Ⅰ和Ⅲ水平检测HeLa细胞瞬时转染后,进行可见光照射和4 Gy X射线照射,24 h后按5 ×107个细胞加入1.0 mL提取液的比例加入提取液,用匀浆器在冰上匀浆,按照试剂盒说明书提取线粒体蛋白,并分别按照试剂盒说明书检测线粒体呼吸链复合物Ⅰ和Ⅲ水平。
1.5 Western blotting法检测细胞中Pink1、parkin、P62和Tom20蛋白表达量HeLa细胞进行瞬时转染后,进行可见光和4 Gy X射线照射,24 h后分别提取总蛋白和线粒体蛋白,并测定蛋白浓度。40 μg蛋白变性后上样,浓缩胶80 V,分离胶120 V,SDS-PAGE电泳后转膜缓冲液4℃中过夜湿转;5%脱脂奶粉封闭1 h后,加入Pink1、parkin、P62和Tom20一抗(采用TBST配置,1:1 000)、GAPDH和HSP60一抗(1:500),37℃孵育2 h,TBST洗涤3次,每次10 min,加入辣根过氧化物酶标记的二抗(1:3 000)后37℃孵育1 h,TBST洗涤3次,ECL试剂盒进行反应,暗室中曝光,并拍照观察目的蛋白表达量。
1.6 统计学分析采用SPSS24.0统计软件进行统计学分析。各组细胞MMP、自噬率和线粒体呼吸链复合物Ⅰ和Ⅲ水平以x±s表示,经正态性检验均符合正态分布,多组间样本均数比较采用方差分析,2组间比较采用Student’ s t检验。以P<0.05表示差异有统计学意义。
2 结果 2.1 各组细胞MMP可见光与对照组比较,照射后24 h,Pink1-mtKR组HeLa细胞MMP明显降低(P<0.05),对照+ 4 GyX射线照射组、空载体+4 GyX射线照射组和Pink1-mtKR+4 GyX射线照射组HeLa细胞MMP降低更明显(P<0.05);与对照+4 GyX射线照射组比较,Pink1-mtKR + 4GyX射线照射组细胞MMP明显降低(P<0.05),且低于Pink1-mtKR组(P<0.05)。见表 1和图 1。
| (n=3, x±s) | |||||||||||||||||||||||||||||
| Group | MMP(MFI) | Mitochondrial respiratory complex Ⅰ [cB/(nmol·L-1)] | Mitochondrial respiratory complexe Ⅲ [cB/(nmol·L-1)] | ||||||||||||||||||||||||||
| Control | 47.01±0.66 | 1.91±0.14 | 0.25±0.02 | ||||||||||||||||||||||||||
| Empty vector | 39.34±2.62 | 1.92±0.18 | 0.25±0.01 | ||||||||||||||||||||||||||
| Pink1-mtKR | 29.03±3.91* | 1.18±0.09* | 0.14±0.07* | ||||||||||||||||||||||||||
| Control+4 Gy X-ray irradiation | 22.18±0.76* | 0.93±0.13* | 0.12±0.02* | ||||||||||||||||||||||||||
| Empty vector+4 Gy X-ray irradiation | 21.41±0.95* | 1.09±0.12* | 0.14±0.04* | ||||||||||||||||||||||||||
| Pink1-mtKR +4 Gy X-ray irradiation | 19.85±1.09*△# | 0.26±0.11**△# | 0.03±0.01**△# | ||||||||||||||||||||||||||
| *P<0.05,**P<0.01 vs control group; △P<0.05 vs control+4 Gy X-ray irradiation group; #P<0.05 vs Pink1-mtKR group. | |||||||||||||||||||||||||||||

|
| A: Control group; B: Empty vector group; C: Pink1-mtKR group; D: Control+4 Gy X-ray irradiation group; E: Empty vector+4 Gy X-ray irradiation group; F: Pink1-mtKR+4 Gy X-ray irradiation group. 图 1 可见光和4 Gy X射线照射后流式细胞术检测各组HeLa细胞MMP Fig. 1 MMP of HeLa cells in various groups detected by flow cytometry after irradiation with visible light and 4 Gy X-ray |
|
|
与对照组比较,可见光照射后24 h,Pink1-mtKR组线粒体呼吸链复合物Ⅰ和Ⅲ水平明显降低(P<0.05),对照+4 GyX射线照射组、空载体+ 4 GyX射线照射组和Pink1-mtKR+4 GyX射线照射组细胞中线粒体呼吸链复合物Ⅰ和Ⅲ水平降低更明显(P<0.05或P<0.01);与对照+4 GyX射线照射组比较,Pink1-mtKR + 4 GyX射线照射组细胞中线粒体呼吸链复合物Ⅰ和Ⅲ水平明显降低(P<0.05),且低于Pink1-mtKR组(P<0.05)。见表 1。
2.3 各组细胞自噬率与对照组比较,可见光照射后12 h,空载体组和Pink1-mtKR组细胞自噬率明显变化(P>0.05);照射后24 h,Pink1-mtKR组细胞自噬率明显升高(P<0.05)。可见光和4 GyX射线联合照射后12和24 h,与对照组比较,对照+4GyX射线照射组、空载体+4GyX射线照射组和Pink1-mtKR+4GyX射线照射组细胞自噬率均明显升高(P<0.05)。与对照+4 Gy X射线照射组比较,照射后24 h,Pink1-mtKR+4 GyX射线照射组细胞自噬率明显升高(P<0.05);照射后12和24 h,Pink1-mtKR +4 GyX射线照射组细胞自噬率明显高于Pink1-mtKR组(P<0.05)。见表 2和图 2。
| (n=3, x±s η/%) | |||||||||||||||||||||||||||||
| Group | Autophagic rate | ||||||||||||||||||||||||||||
| (t/h) 12 | 24 | ||||||||||||||||||||||||||||
| Control | 5.25±0.80 | 6.17±0.38 | |||||||||||||||||||||||||||
| Empty vector | 4.94±0.37 | 6.63±0.93 | |||||||||||||||||||||||||||
| Pink1-mtKR | 6.07±0.20 | 9.01±0.25* | |||||||||||||||||||||||||||
| Control+4 Gy X-ray irradiation | 9.37±0.40* | 20.51±0.51* | |||||||||||||||||||||||||||
| Empty vector+4 Gy X-ray irradiation | 8.80±0.94* | 20.37±0.61* | |||||||||||||||||||||||||||
| Pink1-mtKR+4 Gy X-ray irradiation | 10.91±0.65*# | 27.93±2.54*△# | |||||||||||||||||||||||||||
| *P<0.05 vs control group; △ P<0.05 vs control+4 Gy X-ray irradiation group;P<0.05 vs Pink1-mtKR group. | |||||||||||||||||||||||||||||
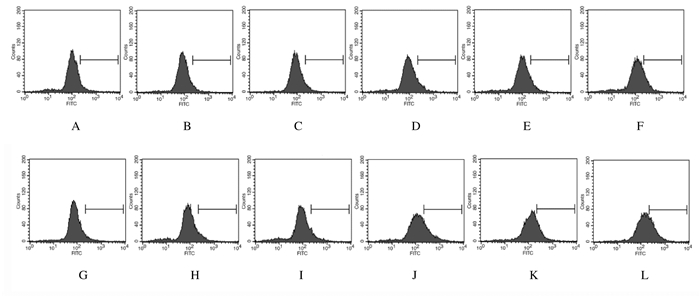
|
| A-F: 12 h; G-L: 24 h; A, G: Control group; B, H: Empty vector group; C, I: Pink1-mtKR group; D, J: Control+4 Gy X-ray irradiation group; E, K: Empty vector+4 Gy X-ray irradiation group; F, L: Pink1-mtKR+4 Gy X-ray irradiation group. 图 2 可见光和X射线照射后12和24 h流式细胞术检测各组HeLa细胞自噬率 Fig. 2 Autophgic rates of HeLa cells in various groups detected by flow cytometry after irradiation with visible light and 4 Gy X-ray |
|
|
在总蛋白中,各组细胞中Pink1、parkin和Tom20蛋白表达量无明显变化,但Pink1-mtKR组P62蛋白表达量增加,Pink1-mtKR+4 GyX射线照射组增加更明显;在线粒体蛋白中,Pink1-mtKR组和Pink1-mtKR + 4 GyX射线照射组细胞中Pink1、parkin和Tom20蛋白表达量均增加。见图 3。
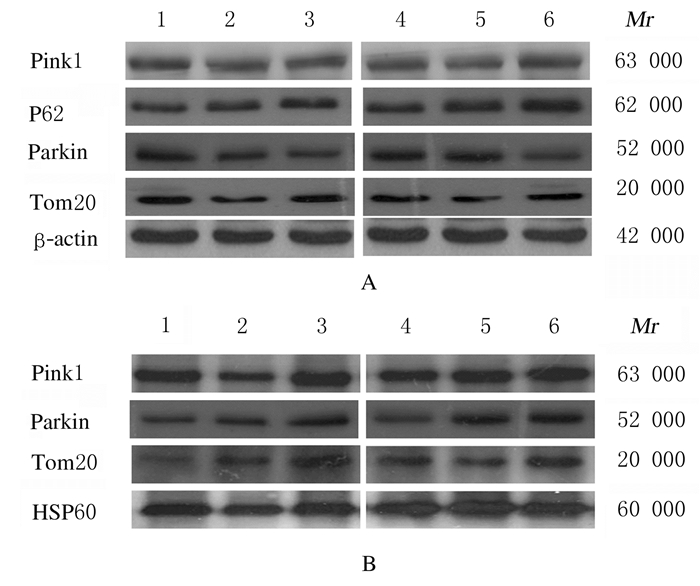
|
| A: Total protein; B: Mitochondrial protein; Lane 1:Control group; Lane 2: Empty vector group; Lane 3: Pink1-mtKR group; Lane 4: Control+4 Gy X-ray irradiationgroup; Lane 5: Empty vector+4 Gy X-ray irradiationgroup; Lane 6: Pink1-mtKR+4 Gy X-ray irradiationgroup. 图 3 Western blotting法检测各组HeLa细胞中自噬相关蛋白表达电泳图 Fig. 3 Electrophoregram of expressions of autophagic proteins in HeLa cells in various groups detected by Western blotting method |
|
|
宫颈癌是妇科常见的恶性肿瘤,对于中晚期宫颈癌患者,放射治疗是首选治疗方式[6-7]。放射治疗主要通过射线的直接作用而损伤细胞;射线间接作用时,主要通过自由基对细胞产生作用。ROS主要由氧化磷酸化途径产生,此过程依赖于线粒体氧化呼吸链复合物Ⅰ和Ⅲ水平。低水平ROS能够发挥细胞正常生理功能,而高水平ROS则诱导氧化应激,损伤线粒体,甚至损伤线粒体DNA[8]。正常生理条件下,ROS可以被抗氧化系统清除,一旦ROS产生过量,则会损伤线粒体和细胞[9-10]。而线粒体是作为细胞主要的能量和代谢源,也是ROS的重要靶点。如何通过靶向线粒体改变其功能而提高治疗肿瘤效果,已引起研究者的广泛关注。基于过量ROS可导致氧化损伤的理论,放疗可以通过诱导ROS而致细胞凋亡和自噬,达到杀伤肿瘤细胞的目的[11-13],大量的实验证据也证明了这一点。如何诱导足量的ROS并且靶向线粒体,最终杀伤肿瘤细胞,是一个重要的研究方向。
研究[4-5]显示:基于线粒体靶向的序列Pink1介导mtKR蛋白,可以实现线粒体靶向诱导ROS产生,且增加辐射诱导的HeLa细胞增殖抑制,可能与促进凋亡有关,但其他机制仍需进一步研究。线粒体主要功能包括产生ATP,90%的ATP由线粒体产生,并在线粒体膜上产生MMP,MMP降低时主要发生线粒体失能表型,而线粒体失能会导致后续的细胞凋亡、自噬和侵袭过程[14-15]。在本研究中,转染了Pink1-mtKR质粒的HeLa细胞中,线粒体呼吸链复合物Ⅰ和Ⅲ水平较对照组和空载体组明显降低,且MMP也明显降低,证明Pink1-mtKR在可见光诱导下可以引起HeLa细胞的线粒体失能。线粒体失能在氧化应激中发挥重要作用,ROS的产生进一步损伤线粒体的电子传递链[16]。
已有文献[17-19]显示:线粒体ROS产生和线粒体脂质的氧化在细胞自噬中起重要作用,是自噬的重要参与者。Pink1存在于大多数线粒体膜中,但通常会降解。当发生线粒体损伤时,Pink1不会降解,会使parkin募集并磷酸化,从而引发线粒体自噬,Pink1敲除小鼠表现出线粒体呼吸活动受损和对氧化应激的敏感性增加[20]。上述结果表明:Pink1/parkin在线粒体自噬和ROS调节的线粒体生成中起作用。许多线粒体自噬的调节因子可以受到ROS的调节,通过ROS诱导MMP降低,可看作是线粒体自噬的一种信号。本研究通过mtKR诱导HeLa细胞线粒体失能,阐明了mtKR在光诱导下导致HeLa细胞自噬的相关机制,即KR靶向线粒体,通过ROS导致MMP去极化,Pink1在线粒体外膜大量累积,招募parkin并使其磷酸化,从而泛素化各种外膜蛋白,进而招募P62等蛋白,以启动线粒体自噬。
本研究结果表明:Pink1-mtKR转染的HeLa细胞经可见光和X射线照射后可以诱导MMP和线粒体呼吸链复合物Ⅰ、Ⅲ水平均降低,mtKR可增加由辐射诱导的细胞自噬,且与Pink1/parkin线粒体自噬途径有关。mtKR引起的线粒体失能和最终的细胞死亡为肿瘤的辐射增敏提供了一个新的思路。
| [1] |
SHIMIZU S, SHINOHARA Y, TSUJIMOTO Y. Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator[J]. Oncogene, 2000, 19(38): 4309-4318. DOI:10.1038/sj.onc.1203788 |
| [2] |
CHOWDHURY S R, SENGUPTA S, BISWAS S, et al. Low fucose containing bacterial polysaccharide facilitate mitochondria-dependent ROS-induced apoptosis of human lung epithelial carcinoma via controlled regulation of MAPKs-mediated Nrf2/Keap1 homeostasis signaling[J]. Mol Carcinog, 2015, 54(12): 1636-1655. DOI:10.1002/mc.22236 |
| [3] |
COSTANTINI P, JACOTOT E, DECAUDIN D, et al. Mitochondrion as a novel target of anticancer chemotherapy[J]. J Natl Cancer Inst, 2000, 92(13): 1042-1053. DOI:10.1093/jnci/92.13.1042 |
| [4] |
李鑫, 马云飞, 唐庚, 等. 线粒体靶向KillerRed诱导的ROS增强辐射对HeLa细胞的增殖抑制作用[J]. 吉林大学学报(医学版), 2018, 44(4): 718-723. |
| [5] |
LI X, FANG F, GAO Y, et al. ROS induced by KillerRed targeting mitochondria (mtKR) enhances apoptosis caused by radiation via cyt c/caspase-3 pathway[J]. Oxid Med Cell Longev, 2019, 2019: 4528616. |
| [6] |
ALLEN J, ROMAY-TALLON R, BRYMER K J, et al. Mitochondria and mood:mitochondrial dysfunction as a key player in the manifestation of depression[J]. Front Neurosci, 2018, 12: 386. DOI:10.3389/fnins.2018.00386 |
| [7] |
WANG W J, LI Y, ZHU J, et al. Prognostic values of systemic inflammation response (SIR) parameters in resectable cervical cancer[J]. Dose Response, 2019, 17(1): 1559325819829543. |
| [8] |
XIE X X, CHEN Y Y, MA L Y, et al. Major depressive disorder mediates accelerated aging in rats subjected to chronic mild stress[J]. Behav Brain Res, 2017, 329: 96-103. DOI:10.1016/j.bbr.2017.04.022 |
| [9] |
ZOROV D B, FILBURN C R, KLOTZ L O, et al. Reactive oxygen species (ROS)-induced ROS release:a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes[J]. J Exp Med, 2000, 192(7): 1001-1014. DOI:10.1084/jem.192.7.1001 |
| [10] |
ZHANG C, ZHAO K L, BU W B, et al. Marriage of scintillator and semiconductor for synchronous radiotherapy and deep photodynamic therapy with diminished oxygen dependence[J]. Angew Chem Int Ed Engl, 2015, 54(6): 1770-1774. DOI:10.1002/anie.201408472 |
| [11] |
DEWAELE M, MAES H, AGOSTINIS P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy[J]. Autophagy, 2010, 6(7): 838-854. DOI:10.4161/auto.6.7.12113 |
| [12] |
BELLOT G L, LIU D, PERVAIZ S. ROS, autophagy, mitochondria and cancer:Ras, the hidden master?[J]. Mitochondrion, 2013, 13(3): 155-162. DOI:10.1016/j.mito.2012.06.007 |
| [13] |
BOL V, BOL A, BOUZIN C, et al. Reprogramming of tumor metabolism by targeting mitochondria improves tumor response to irradiation[J]. Acta Oncol, 2015, 54(2): 266-274. DOI:10.3109/0284186X.2014.932006 |
| [14] |
JÄRÅS M, EBERT B L. Power cut:inhibiting mitochondrial translation to target leukemia[J]. Cancer Cell, 2011, 20(5): 555-556. DOI:10.1016/j.ccr.2011.10.028 |
| [15] |
SHIRAKABE A, ZHAI P Y, IKEDA Y, et al. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure[J]. Circulation, 2016, 133(13): 1249-1263. DOI:10.1161/CIRCULATIONAHA.115.020502 |
| [16] |
SAJADIMAJD S, KHAZAEI M. Oxidative stress and cancer:The role of Nrf2[J]. Curr Cancer Drug Targets, 2018, 18(6): 538-557. DOI:10.2174/1568009617666171002144228 |
| [17] |
SCHERZ-SHOUVAL R, ELAZAR Z. Regulation of autophagy by ROS:physiology and pathology[J]. Trends Biochem Sci, 2011, 36(1): 30-38. DOI:10.1016/j.tibs.2010.07.007 |
| [18] |
ZANDALINAS S I, MITTLER R. ROS-induced ROS release in plant and animal cells[J]. Free Radic Biol Med, 2018, 122: 21-27. DOI:10.1016/j.freeradbiomed.2017.11.028 |
| [19] |
赵艳, 张婷婷. 新型肿瘤靶向纳米颗粒联合光动力治疗对人宫颈癌HeLa细胞的体外杀伤作用[J]. 西安交通大学学报(医学版), 2019, 40(4): 542-548. |
| [20] |
NARENDRA D, TANAKA A, SUEN D F, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy[J]. J Cell Biol, 2008, 183(5): 795-803. DOI:10.1083/jcb.200809125 |
 2020, Vol. 46
2020, Vol. 46


