扩展功能
文章信息
- 赵丽萍, 黄术兵, 张博枰, 周芝兰, 贾雪冰, 孙孟菲, 乔晨萌, 陈雪, 申延琴, 崔春
- ZHAO Liping, HUANG Shubing, ZHANG Boping, ZHOU Zhilan, JIA Xuebing, SUN Mengfei, QIAO Chenmeng, CHEN Xue, SHEN Yanqin, CUI Chun
- 鼠李糖乳杆菌对斑马鱼脊髓损伤后肠道炎症的抑制作用及其机制
- Inhibitory effect of Lactobacillus rhamnosus on intestinal inflammation after spinal cord injury in zebrafishes and its mechanism
- 吉林大学学报(医学版), 2020, 46(04): 680-686
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 680-686
- 10.13481/j.1671-587x.20200403
-
文章历史
- 收稿日期: 2019-10-15
脊髓损伤(spinal cord injury, SCI)是一种严重致残的中枢神经系统损伤,患者常表现为四肢瘫、截瘫[1-2]和神经性肠功能障碍(便秘和大便失禁)等[3]。在大鼠SCI模型中也可见肠道炎症、结肠神经元密度降低[4]和肠道菌群失调[5]等肠道表现,SCI前诱导肠道菌群失调可阻碍运动功能的恢复[6]。因此,SCI引起的肠道病理表现以及肠道菌群对SCI后运动功能恢复和患者生存质量的影响不容忽视。与哺乳动物比较,成年斑马鱼有极强的神经再生能力,在脊髓完全横断后6周即可完全恢复运动能力,是研究SCI的理想动物模型[7]。目前,SCI对斑马鱼肠道炎症和肠道菌群的影响尚无报道。鼠李糖乳杆菌GG株(Lactobacillus rhamnosus GG, LGG)是一种黏着率高、定植力强的益生菌,研究[8]显示:LGG摄入可减轻艾滋病病毒感染者的肠道炎症。但LGG摄入对斑马鱼SCI后肠道炎症和肠道菌群的影响及其机制目前尚无相关报道。本研究探讨了斑马鱼SCI后肠道炎症的发生和LGG对斑马鱼肠道炎症的抑制作用,并从肠屏障和肠道致病菌2个方面阐述了LGG抑制肠道炎症的可能机制。
1 材料与方法 1.1 动物、主要试剂和仪器斑马鱼购于上海佳誉水族馆,斑马鱼饲料购于上海吉荧生物公司。LGG冻干粉购于台湾亚芯公司,MRS琼脂购于青岛海博生物公司,三卡因(MS-222)购于美国Sigma公司,RT-qPCR引物购于上海Invitrogen公司,组织RNA提取试剂盒(R6311-02)购于美国BIOMIGA公司,反转录试剂(RR036A)和荧光定量PCR试剂盒(RR820A)购于日本TaKaRa公司。90mm培养皿购于美国Thermo公司,荧光定量PCR仪(LightCycler 480Ⅱ)购于美国Roche公司,厌氧培养箱(AW200SG)购于英国Electrotek公司,斑马鱼饲养系统购于上海海圣公司,体视显微镜(SMZ-140)购于美国Motic公司,手术器械购于美国WPI公司。
1.2 实验动物饲养条件成年野生型斑马鱼,4~6月龄,雌雄不限, 饲养在实验室室内水循环系统中,水温控制在(28±1)℃,pH值为7.2~7.4,光照周期为14h光照和10 h黑暗(光照时间为8:00至22:00),每天投喂饲料2次(8:30和17:30各1次),每次投喂的饲料量是斑马鱼体质量的1.5%~2.0%。动物实验过程中涉及的所有操作步骤均遵守江南大学动物福利和伦理委员会的相关规定。
1.3 实验动物分组将14条正常斑马鱼随机分为正常饮食组和LGG饮食组(LGG组),每组7条,喂养21 d后取肠道组织。将48条手术斑马鱼随机分为假手术+正常饮食组、假手术+ LGG组、SCI +正常饮食组和SCI + LGG组,每组12条。手术后连续喂养25 d后取肠道组织用于RT-qPCR法检测(每组各6条鱼),喂养7和28 d后斑马鱼取肠道组织用于肠道菌群高通量16s rRNA测序(每组各3条鱼)。正常饮食组斑马鱼喂食商品化饲料;LGG组斑马鱼喂食添加LGG冻干粉的饲料。
1.4 益生菌饲料的制备首先将商品化饲料研磨,再将LGG冻干粉的菌悬液喷洒到饲料上(即LGG饲料),混匀后在20℃无菌条件下风干12h,-20℃保存[9]。正常饮食组饲料不添加LGG,其他流程均与LGG饲料相同。采用平板菌落计数法检测饲料中LGG的浓度,将LGG饲料和正常饮食组饲料样品分别接种到MRS培养基后置于37℃厌氧培养箱内,培养48h后计数。每克饲料中菌落总数(CFU·g-1)=(同一稀释度在3个平皿上形成菌落的平均数×稀释倍数)/取样体积。
1.5 斑马鱼SCI模型制备将斑马鱼置入0.033%的MS-222中麻醉,待腮律动放缓并停止游动时取出,放在体视显微镜下的冰槽中,在鳃盖和背鳍连线的中点处暴露脊髓,然后用精细弹簧剪刀将脊髓一次性剪断(SCI组),SCI后斑马鱼放入含抗真菌剂的水杯中单独饲养。对照组为脊髓假手术斑马鱼(假手术组),即只剪开皮肤和肌肉,但不切断脊髓。
1.6 RT-qPCR法检测斑马鱼肠道组织中肠道屏障相关分子和炎症相关分子mRNA表达水平实验结束后,取各组斑马鱼肠道组织,按照Biomiga EZgeneTM Tissue RNA Kit的说明提取肠道总RNA并测定浓度,然后按PrimeScriptTM RT Master Mix进行反转录,得到cDNA,以该cDNA为模板,按表 1中的引物序列合成相应引物,使用SYBR Premix Ex TaqTM Ⅱ进行RT-qPCR反应。PCR反应条件:95℃、1min;95℃、15s,55℃、15s,72℃、45s,共40个循环。采用2-ΔΔCt法计算斑马鱼肠道组织中肠道屏障相关分子[β防御素样肽-1(defensin beta-like 1, DEFBL1)、紧密连接蛋白2a(tight junction protein 2a, TJP2a)、黏液素2.1 (mucin 2.1, MUC2.1)和黏液素5.3(mucin 5.3, MUC5.3)]和炎症相关分子[肿瘤坏死因子(tumor necrosis factor-α, TNF-α)、Toll样受体2(Toll-like receptor 2, TLR-2)及白细胞介素6(interleukin-6, IL-6)]mRNA表达水平。
| Gene | Primer sequence (5′-3′) |
| TNF-α | F: AGAAGGAGAGTTGCCTTTACCGCT |
| R: AACACCCTCCATACACCCGACTTT | |
| DEFBL1 | F: TGTGCAAGTCTCAGTGGTGTTTGC |
| R: TTTGCCACAGCCTAATGGTCCGAA | |
| MUC2.1 | F: AATATGCCTTGCGGAACAAC |
| R: GTGCTGAGGTTGCAGAATGA | |
| MUC5.3 | F: GGGGAAAACTACACCAGCAA |
| R: TGTGAATTCTGTGCCAGAGC | |
| TLR-2 | F: TGTCTCCCACCCTGAAACTC |
| R: GCCACTCTCCTATCCCAACA | |
| IL-6 | F: TCAACTTCTCCAGCGTGATG |
| R: TCTTTCCCTCTTTTCCTCCTG | |
| TJP2a | F: TGTCTGGAGGACGGGACAAT |
| R: ACTTTGCCACATTTGCGGAG | |
| β-actin | F: AATCTTGCGGTATCCACGAGACCA |
| R: TCTCCTTCTGCATCCTGTCAGCAA |
分别给予正常饮食和LGG饮食的假手术组和SCI组斑马鱼喂养7 d和28d后,取肠道组织及内容物干冰条件下送至上海欧易生物医学科技有限公司,按QIAamp 96 PowerFecal QIAcube HT Kit(5)中的说明提取斑马鱼肠道中的DNA,对样品进行PCR扩增及上机测序分析,通过引物343F(TACGGRAGGCAGCAG)和798R(AGGGTATCTAATCCT)扩增样品16S rRNA的V3-V4区。提取扩增后的高质量序列,通过质量控制过滤去除不符合要求的序列,对得到的优质序列采用操作分类单元(operational taxonomic unit, OTU)进行分类,对各样本中分类到该OTU的序列数(tags)进行统计,获得斑马鱼肠道组织中致病菌属的丰度。
1.8 统计学分析采用SPSS 22.0统计软件进行统计学分析。斑马鱼肠道组织中DEFBL1、MUC2.1、MUC5.3、TNF-α、TLR-2、IL-6和TJP2a mRNA表达水平均符合正态分布,以x±s表示,2组间比较采用t检验,多组间样品均数比较采用单因素方差分析,组间两两比较采用LSD检验。以P<0.05为差异有统计学意义。
2 结果 2.1 饲料中LGG浓度测定为了确定LGG饲料中LGG浓度,采用平板菌落计数法,将LGG饲料和正常饮食组饲料的梯度稀释样品接种到MRS培养基,然后置于37℃厌氧培养箱中培养48 h。结果显示:在LGG饲料的培养皿中散在分布着白色圆形的菌落,菌落直径为1~2 mm,表面光滑、中央有凸起或扁平、质地较软,依据菌落计数结果计算出饲料中LGG浓度为7.3×108 CFU·g-1。正常饮食组饲料无菌落生长。见图 1(插页一)。
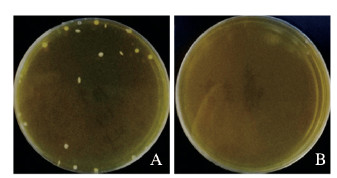
|
| 图 1 LGG组(A)和正常饮食组(B)饲料中LGG菌落形成情况 Fig. 1 Colony formation of LGG in feed in LGG group(A) and normal diet group(B) |
|
|
与正常饮食组比较,LGG组斑马鱼肠道组织中DEFBL1和TJP2a mRNA表达水平明显升高(t=-2.387,P<0.05;t=-12.482,P<0.01),MUC2.1和MUC5.3 mRNA表达水平明显降低(t= 2.497,P<0.05;t= 3.173,P<0.05)。见表 2。
| (n=7, x±s) | |||||||||||||||||||||||||||||
| Group | DEFBL1 mRNA | TJP2a mRNA | MUC2.1 mRNA | Muc5.3 mRNA | |||||||||||||||||||||||||
| Normal diet | 1.00±0.38 | 1.04±0.72 | 1.00±0.23 | 1.00±0.63 | |||||||||||||||||||||||||
| LGG | 1.49±0.25* | 14.72±2.11** | 0.61±0.27* | 0.11±0.04* | |||||||||||||||||||||||||
| *P<0.05, **P<0.01 compared with normal diet group. | |||||||||||||||||||||||||||||
各组斑马鱼肠道组织中TNF-α、TLR-2和IL-6 mRNA表达水平比较差异有统计学意义(TNF-α:F=11.31,P=0.00;TLR-2:F=9.20,P=0.02;IL-6:F=4.35,P=0.02)。与假手术+正常饮食组比较,SCI+正常饮食组斑马鱼肠道组织中TNF-α、TLR-2和IL-6 mRNA表达水平明显升高(P<0.05或P<0.01)。与SCI +正常饮食组比较,SCI + LGG组斑马鱼肠道组织中TNF-α、TLR-2和IL-6 mRNA表达水平明显降低(P<0.05或P<0.01)。见表 3。
| (n=6, x±s) | |||||||||||||||||||||||||||||
| Group | TNF-α mRNA | TLR-2mRNA | IL-6mRNA | ||||||||||||||||||||||||||
| Sham operation+normal diet | 1.00±0.17 | 1.00±0.07 | 1.00±0.27 | ||||||||||||||||||||||||||
| Sham operation+ LGG | 1.04±0.23 | 0.89±0.18 | 0.73±0.16 | ||||||||||||||||||||||||||
| SCI +normal diet | 2.98±0.31** | 2.97±0.59** | 1.95±0.41* | ||||||||||||||||||||||||||
| SCI + LGG | 1.27±0.45△△ | 0.10±0.25△△ | 0.58±0.20△ | ||||||||||||||||||||||||||
| *P<0.05, **P<0.01 compared with sham operation+normal diet group;△ P<0.05, △△ P<0.01 compared with sham operation+LGG group. | |||||||||||||||||||||||||||||
与正常饮食组比较,给予LGG饮食7 d后,斑马鱼肠道组织中致病菌属和条件致病菌属的丰度明显降低,其中致病菌属主要包括气单胞菌属(Aeromonas)和弧菌属(Vibrio),条件致病菌属主要包括脱硫弧菌属(Desulfovibrio)和短波单胞菌属(Brevundimonas); 另外,参与纤维素消化的瘤胃球菌属(Ruminococcaceae)的丰度也明显降低。给予LGG饮食7 d后,斑马鱼肠道组织中Terrimonas、Fastidiosipila和Bryobacter等菌属的丰度均明显升高。
给予LGG饮食28 d后,斑马鱼肠道组织中致病菌属Aeromonas和条件致病菌属Desulfovibrio的丰度明显降低,与LGG饮食处理7 d后的变化趋势一致;而Terrimonas、互营菌属(Syntrophus)、地杆菌属(Geobacter)和Phaselicystis等菌属的丰度则明显升高。LGG饮食组中丰度升高和丰度降低的菌属见表 4和表 5,表中的数字表示分类到该菌属的序列数。
| Group | After 7 d | After 28 d | ||||||
| Aeromonas | Desulfovibrio | Vibrio | Brevundimonas | Ruminococcaceae UCG-014 | Aeromonas | Desulfovibrio | ||
| Sham operation+ normal diet | 1 872 | 107 | 44 | 61 | 8 | 412 | 174 | |
| Sham operation+ LGG | 481 | 87 | 0 | 16 | 0 | 191 | 98 | |
| SCI+normal diet | 8 672 | 188 | 252 | 132 | 38 | 372 | 290 | |
| SCI+LGG | 83 | 54 | 4 | 17 | 20 | 43 | 20 | |
| Group | After 7 d | After 28 d | ||||||
| Terrimonas | Fastidiosipila | Bryobacter | Terrimonas | Syntrophus | Geobacter | Phaselicystis | ||
| Sham operation+ normal diet | 0 | 0 | 0 | 0 | 0 | 0 | 8 | |
| Sham operation+LGG | 3 | 9 | 5 | 6 | 5 | 1 | 10 | |
| SCI+normal diet | 0 | 5 | 5 | 0 | 3 | 0 | 7 | |
| SCI+LGG | 163 | 111 | 121 | 68 | 84 | 53 | 67 | |
肠黏膜屏障由微生物、黏液层(包括黏液素)、抗菌肽(如防御素、溶菌酶和趋化因子等)、肠上皮(包括紧密连接蛋白claudins等)和黏膜免疫系统等几部分组成[10]。研究[11-12]表明:在SCI患者和模型大鼠中均出现了肠黏膜功能丧失或肠屏障破坏。肠道环境的失调也可影响SCI的修复,在大鼠SCI模型中,肠道菌群失调和肠通透性增加,而在损伤前诱导肠道菌群失调阻碍了SCI大鼠运动功能的恢复[6],表明包括肠道菌群在内的肠道环境在SCI恢复过程中起重要作用。因此本研究利用有极强神经再生能力的斑马鱼制备SCI模型,首次探讨了斑马鱼SCI后肠道炎症的发生以及LGG饮食对斑马鱼肠道菌群的影响和减轻肠道炎症的作用机制。
在LGG相关研究中,LGG干预[13-17]的有效浓度范围为1×106CFU·g-1~ 1×1010 CFU·g-1,作用时间为10~33d。在本研究中,LGG饲料中LGG浓度为7.3×108 CFU·g-1,LGG作用时间最短为7 d,最长为28 d。因此本研究中LGG饮食从浓度和饮食时间两个方面均能确保LGG的有效定植。
研究[12]显示:雄性Wistar大鼠T3脊髓损伤后,上段肠道的炎症标记物趋化因子2 (chemokines 2, CCL2)、趋化因子3(chemokines 3, CCL3)和细胞间黏附分子1(intercellular cell adhesion molecule-1, ICAM1)mRNA表达升高,同时伴有小肠组织坏死和肠黏膜绒毛缩短等肠屏障破坏的表现。肠屏障功能的改变与包括炎症性肠病在内的多种疾病有关[10]。在本研究中,首次发现了斑马鱼SCI后肠道组织中炎症因子TNF-α、TLR-2和IL-6mRNA表达水平升高,表明SCI引发了斑马鱼肠道炎症,可能是由于肠屏障功能破坏导致的。研究[18-19]表明:在炎性疾病患者和系统性炎症的动物模型中,口服益生菌能明显降低体内TNF-α的表达水平。新生小鼠灌胃LGG后,肠道内紧密连接蛋白3(claudin 3)表达增加,并且能剂量依赖性地加速肠屏障的成熟[20-21]。体外实验[22]显示:LGG可以阻止大肠杆菌对上皮结构和细胞间紧密连接的破坏,并能通过增加有益菌和保护肠屏障功能,进而减轻高果糖饮食小鼠的肝脏炎症和肝脂肪变性。本研究结果显示:LGG饮食降低了斑马鱼SCI引起的肠道组织中炎症因子表达水平的上升,并且在给予正常斑马鱼LGG饮食21d后,肠屏障相关分子DEFBL1和TJP2a mRNA表达水平明显升高,提示LGG可能通过增强肠上皮屏障功能改善SCI引起的肠道炎症。
研究[23-24]显示:在饮食和化学因素诱导的斑马鱼肠道炎症中,出现了肠黏液层增加,黏膜屏障相关分子如MUC2.2表达水平升高,表明在肠屏障受损的炎症状态下,肠杯状细胞可能会分泌更多的黏液素来保护肠黏膜。在本研究中,LGG饮食组斑马鱼肠道组织中MUC2.1和MUC5.3mRNA表达水平明显降低,可能是因为LGG饮食能增加肠紧密结合蛋白的表达,减少致病菌的定植,塑造了更加健康的肠道状态,但其具体机制仍需进一步研究。
在肠道菌群中,嗜水气单胞菌是引起养殖鱼类肠道炎症的主要条件致病菌,常被用作诱导斑马鱼肠道炎症的实验模型[25]。研究[26-27]表明:LGG能通过凝集素样分子或黏液结合菌毛抑制致病菌(大肠杆菌和粪肠球菌等)的定植。在本研究中,与正常饮食组比较,7 d和28 d后LGG饮食组斑马鱼肠道致病菌丰度明显降低,其中气单胞菌属丰度降低最明显,提示LGG饮食或许能通过抑制致病菌的定植减轻肠道炎症。
综上所述,饮食中有效的LGG浓度和作用时间能够通过影响肠屏障功能和降低肠道内致病菌的丰度减轻斑马鱼SCI引发的肠道炎症,有望能进一步促进斑马鱼SCI后运动功能的恢复。本研究结果为LGG用于减轻SCI患者肠道炎症和调节肠道菌群提供了理论依据。
| [1] |
JACOBS P L, NASH M S. Exercise recommendations for individuals with spinal cord injury[J]. Sports Med, 2004, 34(11): 727-751. |
| [2] |
SCHWAB J M, BRECHTEL K, MUELLER C A, et al. Experimental strategies to promote spinal cord regeneration:an integrative perspective[J]. Prog Neurobiol, 2006, 78(2): 91-116. |
| [3] |
LONGO W E, BALLANTYNE G H, MODLIN I M. The colon, anorectum, and spinal cord patient. A review of the functional alterations of the denervated hindgut[J]. Dis Colon Rectum, 1989, 32(3): 261-267. |
| [4] |
WHITE A R, HOLMES G M. Anatomical and functional changes to the colonic neuromuscular compartment after experimental spinal cord injury[J]. J Neurotrauma, 2018, 35(9): 1079-1090. |
| [5] |
O'CONNOR G, JEFFREY E, MADORMA D, et al. Investigation of microbiota alterations and intestinal inflammation post-spinal cord injury in rat model[J]. J Neurotrauma, 2018, 35(18): 2159-2166. |
| [6] |
KIGERL K A, HALL J C, WANG L L, et al. Gut dysbiosis impairs recovery after spinal cord injury[J]. J Exp Med, 2016, 213(12): 2603-2620. |
| [7] |
PAN H C, LIN J F, MA L P, et al. Major vault protein promotes locomotor recovery and regeneration after spinal cord injury in adult zebrafish[J]. Eur J Neurosci, 2013, 37(2): 203-211. |
| [8] |
ARNBJERG C J, VESTAD B, HOV J R, et al. Effect of Lactobacillus rhamnosus GG supplementation on intestinal inflammation assessed by PET/MRI scans and gut microbiota composition in HIV-infected individuals[J]. J Acquir Immune Defic Syndr, 2018, 78(4): 450-457. |
| [9] |
HARIKRISHNAN R, BALASUNDARAM C, HEO M S. Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV)[J]. Fish Shellfish Immunol, 2010, 29(5): 868-874. |
| [10] |
SÁNCHEZ DE MEDINA F, ROMERO-CALVO I, MASCARAQUE C, et al. Intestinal inflammation and mucosal barrier function[J]. Inflamm Bowel Dis, 2014, 20(12): 2394-2404. |
| [11] |
ZHANG C, ZHANG W H, ZHANG J, et al. Gut microbiota dysbiosis in male patients with chronic traumatic complete spinal cord injury[J]. J Transl Med, 2018, 16(1): 353. |
| [12] |
BESECKER E M, DEITER G M, PIRONI N, et al. Mesenteric vascular dysregulation and intestinal inflammation accompanies experimental spinal cord injury[J]. Am J Physiol Regul Integr Comp Physiol, 2017, 312(1): R146-R156. |
| [13] |
QIN C B, XU L, YANG Y L, et al. Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC6141 and Lactobacillus casei BL23[J]. Reproduction, 2014, 147(1): 53-64. |
| [14] |
SCHNEIDER A C, MACHADO A B, DE ASSIS A M, et al. Effects of Lactobacillus rhamnosus GG on hepatic and serum lipid profiles in zebrafish exposed to ethanol[J]. Zebrafish, 2014, 11(4): 371-378. |
| [15] |
FALCINELLI S, RODILES A, HATEF A, et al. Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish[J]. Sci Rep, 2017, 7(1): 5512. |
| [16] |
VALCARCE D G, RIESCO M F, MARTÍNEZ-VÁZQUEZ J M, et al. Diet supplemented with antioxidant and anti-inflammatory probiotics improves sperm quality after only one spermatogenic cycle in zebrafish model[J]. Nutrients, 2019, 11(4): E843. |
| [17] |
GIOACCHINI G, GIORGINI E, MERRIFIELD D L, et al. Probiotics can induce follicle maturational competence:the Danio rerio case[J]. Biol Reprod, 2012, 86(3): 65. |
| [18] |
D'MELLO C, RONAGHAN N, ZAHEER R, et al. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain[J]. J Neurosci, 2015, 35(30): 10821-10830. |
| [19] |
VAGHEF-MEHRABANY E, ALIPOUR B, HOMAYOUNI-RAD A, et al. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis[J]. Nutrition, 2014, 30(4): 430-435. |
| [20] |
PATEL R M, MYERS L S, KURUNDKAR A R, et al. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function[J]. Am J Pathol, 2012, 180(2): 626-635. |
| [21] |
邓沁, 郭青宝. 双歧杆菌对坏死性小肠结肠炎新生小鼠肠组织的保护作用[J]. 解放军医学杂志, 2020, 45(1): 49-54. |
| [22] |
RITZE Y, BÁRDOS G, CLAUS A, et al. Lactobacillus rhamnosus GG protects against non-alcoholic fatty liver disease in mice[J]. PLoS One, 2014, 9(1): e80169. |
| [23] |
ULLOA P E, SOLÍS C J, DE LA PAZ J F, et al. Lactoferrin decreases the intestinal inflammation triggered by a soybean meal-based diet in zebrafish[J]. J Immunol Res, 2016, 2016: 1639720. |
| [24] |
OEHLERS S H, FLORES M V, HALL C J, et al. Retinoic acid suppresses intestinal mucus production and exacerbates experimental enterocolitis[J]. Dis Model Mech, 2012, 5(4): 457-467. |
| [25] |
HU Z Y, WU B Q, MENG F H, et al. Impact of molecular hydrogen treatments on the innate immune activity and survival of zebrafish (Danio rerio) challenged with Aeromonas hydrophila[J]. Fish Shellfish Immunol, 2017, 67: 554-560. |
| [26] |
PETROVA M I, IMHOLZ N C, VERHOEVEN T L, et al. Lectin-like molecules of lactobacillus rhamnosus GG inhibit pathogenic escherichia coli and salmonella biofilm formation[J]. PLoS One, 2016, 11(8): e0161337. |
| [27] |
TYTGAT H L, DOUILLARD F P, REUNANEN J, et al. Lactobacillus rhamnosus GG outcompetes enterococcus faecium via mucus-binding pili:evidence for a novel and heterospecific probiotic mechanism[J]. Appl Environ Microbiol, 2016, 82(19): 5756-5762. |
 2020, Vol. 46
2020, Vol. 46


