扩展功能
文章信息
- 周宁, 武睿, 马振凯, 陈微微, 李雪琳, 宫凯凯, 杨丽娟, 代娟娟, 武艳
- ZHOU Ning, WU Rui, MA Zhenkai, CHEN Weiwei, LI Xuelin, GONG Kaikai, YANG Lijuan, DAI Juanjuan, WU Yan
- 下调ADAR1表达对人肺癌细胞增殖、迁移和上皮间质转化的影响
- Effects of down-regulation of ADAR1 expression on proliferation, migration and epithelial-mesenchymal transition of human lung cancer cells
- 吉林大学学报(医学版), 2020, 46(04): 669-674
- Journal of Jilin University (Medicine Edition), 2020, 46(04): 669-674
- 10.13481/j.1671-587x.20200401
-
文章历史
- 收稿日期: 2019-12-10
2. 滨州医学院附属医院肿瘤研究实验室, 山东 滨州 256600;
3. 山东省滨州市人民医院神经外科, 山东 滨州 256600
2. Cancer Research Institute, Affiliated Hospital, Binzhou Medical University, Binzhou 256603, China;
3. Department of Neurosurgery, Binzhou People's Hospital, Binzhou 256603, China
肺癌的发生发展与异常的基因表达调控有密切关联,RNA编辑是一种重要的基因转录后修饰方式,在调控基因表达中发挥重要作用。作用于RNA的腺苷酸脱氢酶1(adenosine deaminase acting on RNA enzyme 1,ADAR1)能够催化RNA上腺嘌呤转化为次黄嘌呤(A-I),并将次黄嘌呤识别为鸟嘌呤,与胞嘧啶进行配对,最后导致蛋白质中氨基酸的改变,是一种重要的RNA编辑酶[1]。ADAR1的主要功能是编辑内源的双链核糖核酸(double-stranded RNA,dsRNA)使之与病毒来源的dsRNA区分开来[2]。研究[3-4]表明:缺失ADAR1的造血干细胞导致干扰素刺激基因(interferon-stimulated genes,ISGs)表达上调,ADAR1在免疫应答中起重要作用。ADAR1还具有非依赖编辑的功能,例如能够防止转录本的降解。双链RNA结合蛋白staufen1和staufen2与mRNA的结合能招募上游移码突变体(up-frameshift mutant 1,UPF1),并能激活staufen进而促进RNA降解(SMD)[5-6]。ADAR1表达水平变化还与黑色素瘤[7]、食道癌[8]、慢性白血病[9]和宫颈癌[10]等的发生发展有密切关联。有文献[11]报道:ADAR1通过增强局部黏着斑激酶(focal adhesion kinase,FAK)mRNA的稳定性促进肺腺癌细胞的迁移和浸润。但敲低ADAR1对肺癌细胞增殖和上皮间质转化(epithelial-mesenchymal transition,EMT)的调控机制尚无报道。本研究利用RNA干扰技术敲低细胞中ADAR1表达水平,建立了稳定表达shADAR1的H1299和H520细胞系,并通过细胞增殖、克隆形成、细胞划痕实验及Western blotting法评价ADAR1对细胞增殖、迁移和EMT的调节作用,以期为研究ADAR1在肺癌发展中的作用机制奠定基础,同时为以ADAR1为靶点的抗癌药物设计提供理论依据。
1 材料与方法 1.1 细胞、主要试剂和仪器人肺癌H1299和H520细胞购自美国模式培养物研究所(ATCC)。胰蛋白酶、胎牛血清(FBS)和RPMI 1640培养基均购自美国Gibco公司,shADAR1和对照质粒(negative shRNA)购自上海吉凯基因化学技术有限公司,Lipofectamine 2000购于美国Invitrogen公司,TRIzol试剂和PCR所需引物购于上海生物工程有限公司,逆转录试剂购于Thermo公司,ADAR1单克隆抗体、Tubulin抗体和辣根过氧化物酶标记的羊抗兔IgG抗体购于美国Proteintech公司,波形蛋白(vimentin)抗体和E钙黏蛋白(E-cadherin)抗体购自美国Abcam公司,CCK-8试剂购于上海碧云天公司。CO2细胞培养箱(美国Thermo公司),激光共聚焦显微镜(美国Leica公司),酶标仪(美国Bio-Tek公司)。
1.2 细胞转染和稳定细胞系的构建细胞用含10%胎牛血清和1%(V/V)青霉素-链霉素的RPMI-1640培养基于37℃、5% CO2及饱和湿度环境的培养箱中培养。H1299和H520细胞均分为对照组和shADAR1组,分别将1.5 μg negative shRNA质粒或shADAR1质粒与Lipofectamine 2000 4 μL混合后,加入培养基100 μL,室温孵育25 min,分别加入到生长密度为60%~80%的H1299和H520细胞中,继续培养24~48 h后,以野生型细胞为平行对照进行嘌呤霉素筛选,药物浓度为2 mg·L-1,2周后平行对照细胞全部死亡,并且荧光显微镜下转染后细胞全部发出绿色荧光,即稳定细胞系构建成功,后续改为嘌呤霉素半量维持培养。
1.3 实时荧光定量PCR(qRT-PCR)法检测细胞中ADAR1 mRNA表达水平收集各组细胞,TRIzol法提取细胞总RNA,将提取的RNA应用逆转录试剂盒逆转录成cDNA,应用TransStart Green qPCR SuperMix进行PCR验证。qRT-PCR的反应条件:95 ℃、30 s预变性;95 ℃、5 s,60 ℃、30 s,共40个循环。引物序列:ADAR1,上游引物为5′-CCCTTCAGCCACATCCTTC-3′,下游引物为5′-GCCATCTGCTTTGCCACTT-3′;GAPDH,上游引物为5′-GGTGGTCTCCTCTGACTTCAACA-3′,下游引物为5′- GTTGCTGTAGCCAAATTCGTTGT-3′。进行3次重复实验,以GAPDH作为内参,采用2-ΔΔCt法计算ADAR1 mRNA相对表达水平。
1.4 CCK-8法检测细胞增殖活性将各组细胞以5×103 /孔接种于96孔板,每组设置3个平行孔,每过12 h加入CCK-8继续培养3 h,采用酶标仪测定各孔在450 nm波长处的吸光度(A)值,共测定至72 h,实验重复3次,取平均A值,以A值代表细胞增殖活性,对0 h平均A值进行均一化处理后,采用GraphPad Prism 7.0软件绘制各组细胞随时间变化的增殖曲线。
1.5 克隆形成实验检测细胞的克隆形成能力将各组细胞以1×103个/孔的密度接种于6孔板中,每组设2个复孔。培养14 d后,吸尽培养基并用PBS清洗细胞,加入4%多聚甲醛固定5 min,再用0.1%结晶紫染液染色15 min,用PBS清洗后拍照并计算克隆形成数,代表细胞的克隆形成能力。实验重复3次。
1.6 划痕实验检测细胞划痕愈合率各组细胞培养过夜后,用200 μL枪头进行划痕,PBS洗涤后,加入新鲜无血清培养基后继续培养,分别在0和48 h进行拍照。实验重复3次,应用Image J软件对划痕距离进行统计学分析,并采用GraphPad Prism7.0软件绘制各组细胞的划痕愈合率图。细胞划痕愈合率=(0 h划痕间距-48 h划痕间距)/0 h划痕间距×100%。
1.7 Western blotting法检测细胞中ADAR1、E-cadherin和vimentin蛋白表达水平收集各组H1299和H520细胞,NP-40裂解液裂解细胞,BCA法测定浓度后,通过10% SDS-PAGE进行电泳,转膜,封闭后分别孵育抗ADAR1(1:1 000)、抗Tubulin(1:1 000)、抗vimentin(1:1 000)和抗E-cadherin(1:1 000)抗体过夜,次日PBST洗涤3次,孵育HRP标记的羊抗兔或羊抗鼠IgG抗体(1:1 000),加入ECL显色底物,应用成像系统进行成像拍照,实验重复3次。利用ImageJ分析软件分析各条带灰度值,以目的蛋白条带灰度值/Tubulin蛋白条带灰度值比值作为目的蛋白表达水平。
1.8 统计学分析采用SPSS12.0统计软件进行统计学分析。采用Shapiro-Wilk检验验证各组数据的正态分布情况。各组细胞中ADAR1 mRNA表达水平、细胞增殖活性、克隆形成数量、细胞划痕愈合率和细胞中蛋白表达水平均呈正态分布,以x±s表示,2组间样本均数比较采用t检验,2种细胞不同时间点样本均数比较采用双因素方差分析,各时间点间两两比较采用Turkey检验。以P<0.05为差异有统计学意义。
2 结果 2.1 敲低ADAR1稳定细胞系的构建和鉴定将携带有绿色荧光蛋白(green fluorescent protein, GFP)基因的shADAR1载体和对照质粒分别瞬时转染H1299及H520细胞后,应用荧光显微镜检测GFP的表达情况,以此检测脂质体的转染效率。结果显示:H1299和H520细胞中转染对照质粒和shADAR1后,90%以上细胞中均可见绿色荧光。见图 1(插页一)。
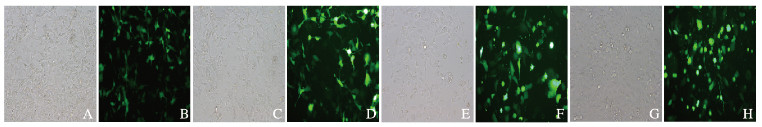
|
| A, B, E, F: Control group; C, D, G, H: shADAR1 group; A, C, E, G: Bright field imaging; B, D, F, H: Fluorescence microscope. 图 1 荧光显微镜观察各组H1299细胞(A~D)和H520细胞(E~H)转染效率(×40) Fig. 1 Transfection efficiencies of H1299 cells (A —D) and H520 cells (E—H) in various groups observed by fluorescence microscope(× 40) |
|
|
与对照组(1.000±0.081和1.000±0.040)比较,shADAR1组H1299和H520细胞中ADAR1 mRNA表达水平(0.530±0.040和0.405±0.045)均明显降低(t=5.255,P<0.05;t=9.882,P<0.01)。Western blotting法检测结果显示:shADAR1组H1299和H520细胞中ADAR1蛋白表达水平(0.296±0.014和0.371± 0.052)明显低于对照组(1.000±0.126和1.000± 0.043)(P<0.05)。见图 2。
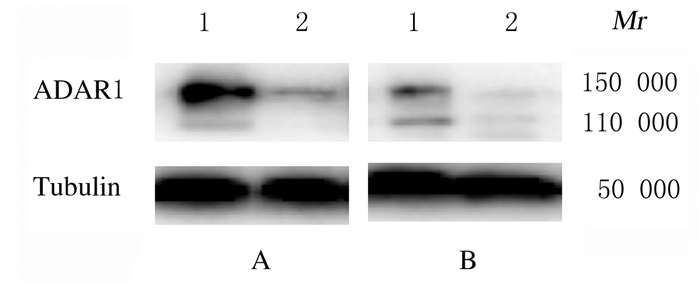
|
| A:H1299 cells; B:H520 cells; Lane 1: Control group; Lane 2: shADAR1 group. 图 2 Western blotting法检测各组细胞中ADAR1蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of ADAR1 protein in cells in various groups detected by Western blotting method |
|
|
与对照组比较,shADAR1组H1299和H520细胞增殖活性明显降低(H1299:t=3.106,P<0.05;H520:t=3.255,P<0.01)。见图 3。
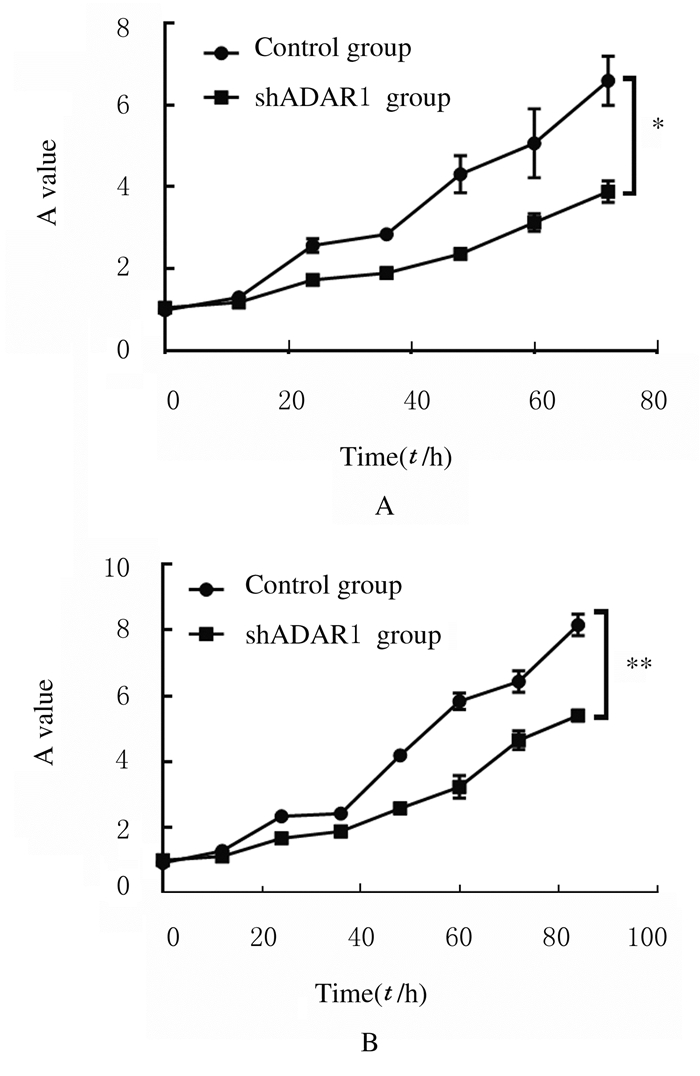
|
| A: H1299 cells; B: H520 cells. *P < 0.05, **P < 0.01 compared with control group. 图 3 各组H1299和H520细胞增殖活性 Fig. 3 Proliferation activities of H1299 and H520 cells in various groups |
|
|
与对照组(172.500±10.607和213.500±6.364)比较,shADAR1组H1299和H520细胞克隆形成数(38.500±6.364和59.500±4.950)明显降低(t=14.58,P<0.01;t=16.75,P<0.01),即克隆形成能力降低。见图 4。
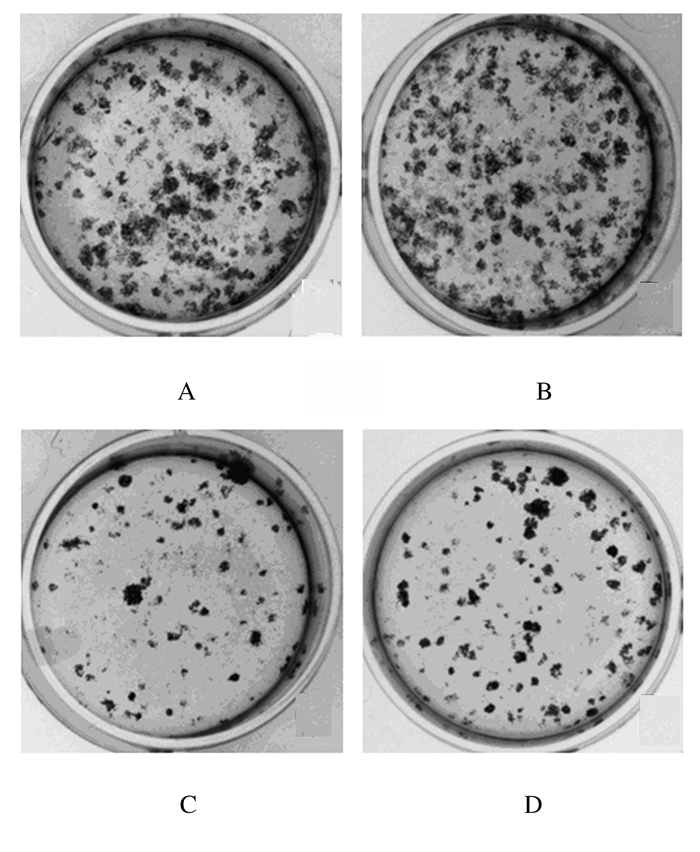
|
| A, B:Control group; C, D: shADAR1 group; A, C: H1299 cells; B, D: H520 cells. 图 4 各组H1299和H520细胞克隆形成情况 Fig. 4 Colony formation of H1299 and H520 cells in various groups |
|
|
与对照组(59.1%±8.7%和57.7%±1.7%)比较,shADAR1组H1299和H520细胞划痕愈合率(43.6%±8.9%和45.1%±5.0%)明显降低(t=3.043,P<0.01;t=4.149,P<0.01)。见图 5。
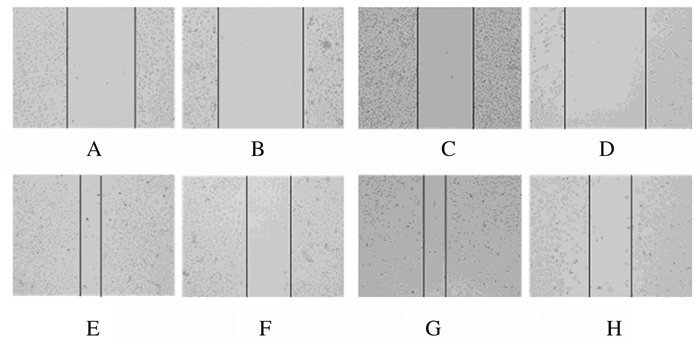
|
| A-D:0 h; E-H:48 h; A, B, E, F:H1299 cells; C, D, G, H:H520 cells; A, C, E, G:Control group; B, D, F, H:shADAR1 group. 图 5 划痕实验检测各组H1299和H520细胞划痕愈合率 Fig. 5 Scratch healing rates of H1299 and H520 cells in various groups detected by scratch assay |
|
|
shADAR1组H1299和H520细胞中E-cadherin蛋白表达水平(1.700±0.018和2.481±0.152)明显高于对照组(1.000±0.070和1.000±0.084)(t=13.8,P<0.01;t=12.02,P<0.01)。
shADAR1组H1299和H520细胞中vimentin蛋白表达水平(0.160±0.009和0.336±0.049)明显低于对照组(1.000±0.056和1.000±0.056)(t=20.68,P<0.01;t=12.49,P<0.01)。见图 6。

|
| A:H1299 cells; B:H520 cells; Lane 1: Control group; Lane 2: shADAR1 group. 图 6 Western blotting法检测各组H1299和H520细胞中E-cadherin和vimentin蛋白表达电泳图 Fig. 6 Electrophoregram of expressions of E-cadherin and vimentin proteins in H1299 and H520 cells in various groups detected by Western blotting method |
|
|
化疗在非小细胞肺癌治疗中占重要地位,但是多数的治疗效果并不理想,主要原因是癌细胞在治疗过程中产生了抗药性。因此,研究肺癌发生发展中的关键调控因子,发现新的治疗靶点对肺癌的治疗具有重要意义。
RNA编辑主要指在转录本上碱基的插入、缺失或者替换,是一种重要的转录后修饰[12],其中A-to-I(也称为A-to-G编辑)是哺乳动物中最常见的RNA编辑方式。ADAR1是一种重要RNA编辑酶,存在2种单体形式,分别为ADAR1p110和ADAR1p150[13]。ADAR1相互作用的蛋白主要分为两类,一类是早期发现能被ADAR1编辑的相互作用蛋白,如丙酮酸激酶(pyruvate kinase,PKR)[14];另一类是不依赖编辑功能,直接与ADAR1相互作用的蛋白,如核因子(nuclear factor,NF)45[15]和NF90[16],随后还发现移码突变蛋白1(Upf1)[17]和Dice酶[18],均可与ADAR1相互作用,在机体内发挥重要的生物学功能。
ADAR1除在免疫应答中的重要作用之外,在多种肿瘤中存在过表达的现象,并在肿瘤发生过程中发挥重要的调控作用,例如在肝癌中,ADAR1的表达水平上调后能稳定抗酶抑制因子1(antizyme inhibitor 1,AZIN1)的水平,导致下游原癌基因表达上调[19]。在结直肠癌中,Ras同源基因家族成员Q(ras homolog family member Q,RHOQ)的A-to-Ⅰ的编辑能够增加肿瘤细胞的侵袭潜能[20]。异常表达的ADAR1通过激活哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)/p70S6K信号通络调控胃癌的发生发展[21]。本研究通过RNA干扰技术并通过抗生素筛选获得稳定低表达ADAR1细胞系,并通过CCK-8实验、克隆形成及划痕实验证明:敲低ADAR1能够抑制H1299和H520细胞的增殖和迁移能力。本研究结果与文献[22]报道的异常表达的ADAR1促进肺癌的发生发展过程的结果一致。最近研究[23]显示:异常表达的ADAR1通过提高c-myc的稳定性介导了胰腺癌细胞对布罗莫结构域和外末端基元蛋白(bromodomain and extraterminal motif,BET)的抵抗性。上述结果说明:ADAR1作为一种促癌基因在肺癌的发生发展中发挥重要作用,也可能参与了肿瘤耐药性的产生。
EMT是肿瘤细胞迁移浸润的重要途径,E-cadherin表达下调、vimentin表达上调是EMT的特征之一[24]。E盒结合锌指蛋白1(zinc finger E-box-binding homeobox 1,ZEB1)[25]、锌指转录因子2(zinc finger transcription factor 2,snail2)[26]等在转录水平上抑制E-cadherin的表达,促进细胞的EMT转化。EMT能够促进癌细胞的转移、浸润和药物抵抗。本研究结果表明:敲低ADAR1可激活E-cadherin,并抑制vimentin的表达,抑制H1299和H520细胞的EMT过程,但ADAR1调控EMT的具体作用机制还需进一步深入研究。
综上所述,本研究首先通过RNA干扰技术抑制细胞中ADAR1表达,并进一步验证敲低ADAR1后可抑制肺癌H1299和H520细胞的增殖、迁移和EMT。本研究结果为肺癌的发病机制和治疗靶点的研究提供了理论依据。
| [1] |
PULLIRSCH D, JANTSCH M F. Proteome diversification by adenosine to inosine RNA editing[J]. RNA Biol, 2010, 7(2): 205-212. DOI:10.4161/rna.7.2.11286 |
| [2] |
LIDDICOAT B J, PISKOL R, CHALK A M, et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself[J]. Science, 2015, 349(6252): 1115-1120. DOI:10.1126/science.aac7049 |
| [3] |
HARTNER J C, WALKLEY C R, LU J, et al. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling[J]. Nat Immunol, 2009, 10(1): 109-115. |
| [4] |
MANNION N M, GREENWOOD S M, YOUNG R, et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA[J]. Cell Rep, 2014, 9(4): 1482-1494. |
| [5] |
KIM Y K, FURIC L, DESGROSEILLERS L, et al. Mammalian Staufen1 recruits Upf1 to specific mRNA 3'UTRs so as to elicit mRNA decay[J]. Cell, 2005, 120(2): 195-208. |
| [6] |
PARK E, GLEGHORN M L, MAQUAT L E. Staufen2 functions in Staufen1-mediated mRNA decay by binding to itself and its paralog and promoting UPF1 helicase but not ATPase activity[J]. Proc Natl Acad Sci U S A, 2013, 110(2): 405-412. DOI:10.1073/pnas.1213508110 |
| [7] |
SHOSHAN E, MOBLEY A K, BRAEUER R R, et al. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis[J]. Nat Cell Biol, 2015, 17(3): 311-321. DOI:10.1038/ncb3110 |
| [8] |
QIN Y R, QIAO J J, CHAN T H, et al. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma[J]. Cancer Res, 2014, 74(3): 840-851. |
| [9] |
JIANG Q, CREWS L A, BARRETT C L, et al. ADAR1 promotes malignant progenitor reprogramming in chronic myeloid leukemia[J]. Proc Natl Acad Sci USA, 2013, 110(3): 1041-1046. DOI:10.1073/pnas.1213021110 |
| [10] |
CHEN Y, WANG H, LIN W Y, et al. ADAR1 overexpression is associated with cervical cancer progression and angiogenesis[J]. Diagn Pathol, 2017, 12(1): 12. |
| [11] |
AMIN E M, LIU Y, DENG S, et al. The RNA-editing enzyme ADAR promotes lung adenocarcinoma migration and invasion by stabilizing FAK[J]. Sci Signal, 2017, 10(497): eaah3941. DOI:10.1126/scisignal.aah3941 |
| [12] |
BASS B L. RNA editing by adenosine deaminases that act on RNA[J]. Annu Rev Biochem, 2002, 71: 817-846. DOI:10.1146/annurev.biochem.71.110601.135501 |
| [13] |
HEEP M, MACH P, REAUTSCHNIG P, et al. Applying human ADAR1p110 and ADAR1p150 for site-directed RNA editing-G/C substitution stabilizes GuideRNAs against editing[J]. Genes (Basel), 2017, 8(1): E34. DOI:10.3390/genes8010034 |
| [14] |
LIU Y, LEI M, SAMUEL C E. Chimeric double-stranded RNA-specific adenosine deaminase ADAR1 proteins reveal functional selectivity of double-stranded RNA-binding domains from ADAR1 and protein kinase PKR[J]. Proc Natl Acad Sci U S A, 2000, 97(23): 12541-12546. DOI:10.1073/pnas.97.23.12541 |
| [15] |
QUINONES-VALDEZ G, TRAN S S, JUN H I, et al. Regulation of RNA editing by RNA-binding proteins in human cells[J]. Commun Biol, 2019, 2: 19. DOI:10.1038/s42003-018-0271-8 |
| [16] |
NIE Y, DING L, KAO P N, et al. ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing[J]. Mol Cell Biol, 2005, 25(16): 6956-6963. DOI:10.1128/MCB.25.16.6956-6963.2005 |
| [17] |
AGRANAT L, RAITSKIN O, SPERLING J, et al. The editing enzyme ADAR1 and the mRNA surveillance protein hUpf1 interact in the cell nucleus[J]. Proc Natl Acad Sci U S A, 2008, 105(13): 5028-5033. DOI:10.1073/pnas.0710576105 |
| [18] |
OTA H, SAKURAI M, GUPTA R, et al. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing[J]. Cell, 2013, 153(3): 575-589. |
| [19] |
CHEN L, LI Y, LIN C H, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma[J]. Nat Med, 2013, 19(2): 209-216. DOI:10.1038/nm.3043 |
| [20] |
HAN S W, KIM H P, SHIN J Y, et al. RNA editing in RHOQ promotes invasion potential in colorectal cancer[J]. J Exp Med, 2014, 211(4): 613-621. DOI:10.1084/jem.20132209 |
| [21] |
DOU N, YU S, YE X, et al. Aberrant overexpression of ADAR1 promotes gastric cancer progression by activating mTOR/p70S6K signaling[J]. Oncotarget, 2016, 7(52): 86161-86173. DOI:10.18632/oncotarget.13354 |
| [22] |
ANADÓN C, GUIL S, SIMÓ-RIUDALBAS L, et al. Gene amplification-associated overexpression of the RNA editing enzyme ADAR1 enhances human lung tumorigenesis[J]. Oncogene, 2016, 35(33): 4422. |
| [23] |
SUN Y, FAN J, WANG B, et al. The aberrant expression of ADAR1 promotes resistance to BET inhibitors in pancreatic cancer by stabilizing c-Myc[J]. Am J Cancer Res, 2020, 10(1): 148-163. |
| [24] |
CHEN T, YOU Y N, JIANG H, et al. Epithelial-mesenchymal transition (EMT):a biological process in the development, stem cell differentiation, and tumorigenesis[J]. J Cell Physiol, 2017, 232(12): 3261-3272. DOI:10.1002/jcp.25797 |
| [25] |
MA P, NI K, KE J, et al. miR-448 inhibits the epithelial-mesenchymal transition in breast cancer cells by directly targeting the E-cadherin repressor ZEB1/2[J]. Exp Biol Med (Maywood), 2018, 243(5): 473-480. DOI:10.1177/1535370218754848 |
| [26] |
PARK J J, PARK M H, OH E H, et al. The p21-activated kinase 4-Slug transcription factor axis promotes epithelial-mesenchymal transition and worsens prognosis in prostate cancer[J]. Oncogene, 2018, 37(38): 5147-5159. DOI:10.1038/s41388-018-0327-8 |
 2020, Vol. 46
2020, Vol. 46


