扩展功能
文章信息
- 张铂彦, 张悦, 刘贺, 吴乃超, 王金成
- COL2A1基因突变与Ⅱ型胶原蛋白病表型关系的研究进展
- Research progress in relationship between COL2A1 gene mutations and phenotypes of type Ⅱ collagenopathies
- 吉林大学学报(医学版), 2020, 46(03): 643-648
- Journal of Jilin University (Medicine Edition), 2020, 46(03): 643-648
- 10.13481/j.1671-587x.20200335
-
文章历史
- 收稿日期: 2019-08-17
2. 吉林大学第一医院放疗科, 吉林 长春 130021
COL2A1基因编码Ⅱ型胶原前体蛋白α1链,3条完全相同的α1链相互折叠后能够形成前体蛋白同型三聚体,同型三聚体再经交联作用构成成熟的Ⅱ型胶原蛋白[1]。在α1链中存在一段特殊的三重螺旋区,该区域特征序列为重复的Gly-X-Y序列(Gly为甘氨酸,X和Y表示其他氨基酸),BARAT-HOUARI等[2]研究显示:三重螺旋结构对胶原蛋白分子的稳定性起重要作用。
COL2A1基因突变会改变基因序列-蛋白结构-蛋白功能关系轴,进而导致一系列疾病[3],统称为Ⅱ型胶原蛋白病(OMIM编号:120140),该疾病主要为常染色体显性遗传病。本研究基于《莱顿突变位点开放式数据库》(LOVD)以及最新研究报道,汇总获得COL2A1基因突变所致的21种疾病亚型,以及全部基因突变位点、突变类型和临床表型,并汇总患者年龄、性别和国籍信息。基于以上信息,本研究对COL2A1基因型和表型的关系进行探讨和分析。
近年来有关COL2A1基因突变的研究在国内外均可见较多报道,但综述类报道较少,且综述类报道均未对COL2A1基因突变类型进行汇总分析亦未对Ⅱ型胶原蛋白病进行总结分类。本研究旨在帮助临床医生深入了解COL2A1基因突变导致的Ⅱ型胶原蛋白病的复杂临床表型,推进基因筛查在临床中的应用,从而进一步扩大COL2A1基因突变的表型谱。
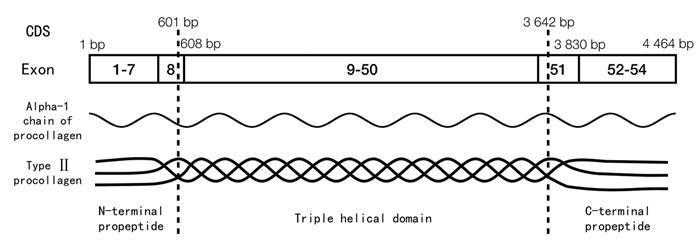
1 COL2A1基因突变的位置和类型 1.1 突变位置COL2A1基因长度为33 246 bp,包含54个外显子,蛋白质编码区(coding sequence, CDS)区域总长度为4 464 bp,三重螺旋区位于CDS的601~3 642 bp,其N末端前肽区对应1~608 bp,C末端前肽区对应3 643~4 464 bp。COL2A1基因和Ⅱ型胶原蛋白结构示意图见图 1。
|
| 图 1 COL2A1基因和Ⅱ型胶原蛋白示意图 Fig. 1 Diagram of illustration of COL2A1 gene and type Ⅱ collagen |
|
|
本研究汇总的657例COL2A1基因突变病例中,有535例COL2A1基因突变发生在CDS区,有117例发生在内含子区,还有5例大片段基因缺失的情况。在CDS区发生突变的病例中,有437例突变位点发生在三重螺旋区,49例发生在N末端前肽区,49例发生在C末端前肽区。
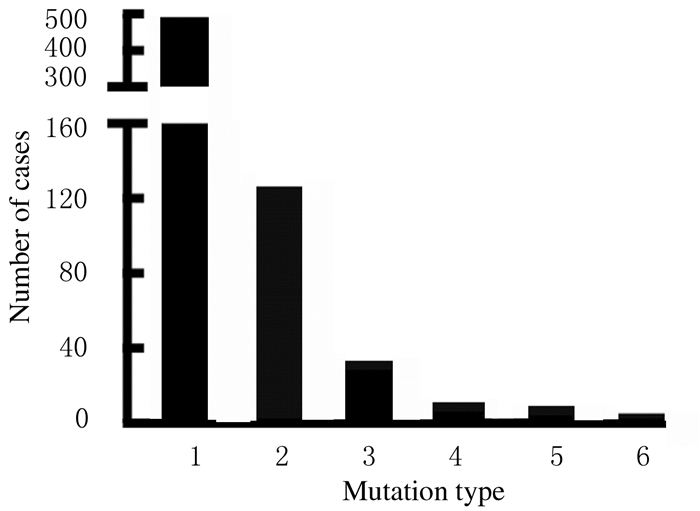
1.2 COL2A1基因突变类型和突变效果COL2A1基因突变中最多为点突变(substitution),约占73.4%(n=657),其次是小片段删除(小于20 bp的片段删除)占19.0%,复制(duplication)占4.7%,缺失插入(delins)占1.4%,大片段删除占1.1%,插入(insertion)占0.5%。见图 2。
|
| 1:Substitution; 2:Small deletion; 3:Duplicaton; 4:Delins; 5:Large deletion; 6:Insertion. 图 2 本研究中COL2A1突变类型 Fig. 2 Mutation types percentage of COL2A1 in our study |
|
|
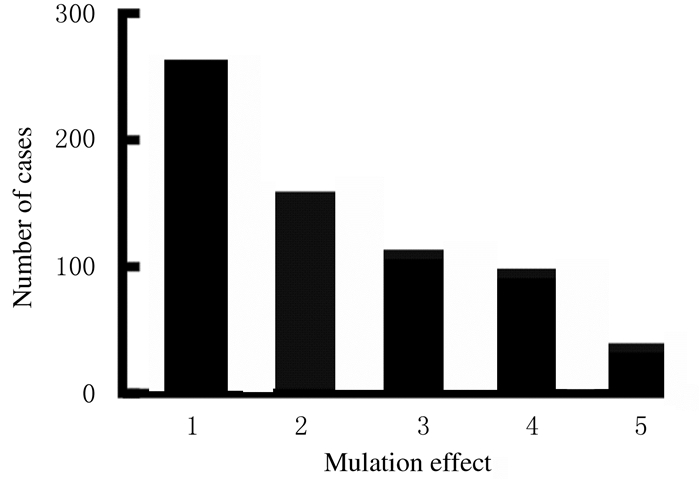
从突变效果的角度分析,错义突变占比最高(39.6%),其次是移码突变(23.7%)、无义突变(16.7%)和发生在内含子的突变(14.5%)等。见图 3。
|
| 1:Missense; 2:Framehift; 3:Nonsense; 4:RNA processing; 5:Other mutations. 图 3 本研究中COL2A1突变效果 Fig. 3 Mutation effects of COL2A1 in our study |
|
|
COL2A1基因突变会导致一系列以软骨发育异常和视听障碍为特征的表型,致病机制为COL2A1基因所编码的Ⅱ型胶原蛋白是透明软骨(如肋软骨、气管软骨)和关节软骨(如膝关节、髋关节),Ⅱ型胶原蛋白结构异常会影响软骨的正常生理功能[4]。
2.1 COL2A1基因突变患者的年龄、性别和国籍分布本研究汇总385例患者的年龄信息,平均确诊年龄为15.32岁,确诊患者年龄最低的表型为Ⅱ型软骨发育不全(0.53岁),其为“致死型”表型;确诊患者年龄最高的表型是股骨头缺血性坏死(30.29岁),其为“轻型”表型,故可推测确诊患者年龄与疾病严重程度存在相关性。
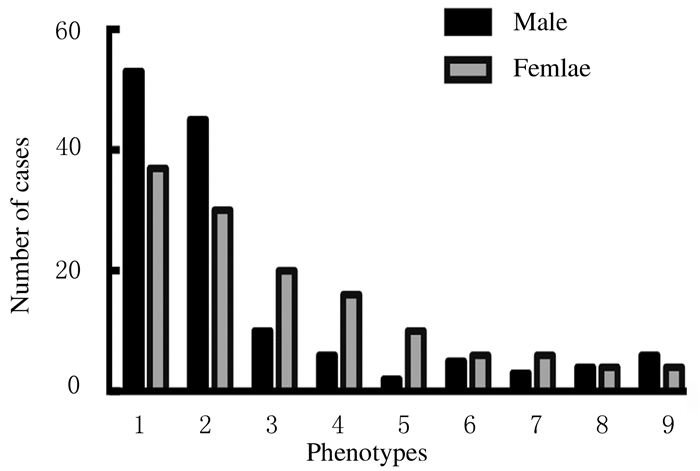
在294例患者的性别信息中,男性150例,女性144例,各表型的性别分布见图 4。在657例患者国籍信息中,病例报道较多的国家分别是英国(167例)、比利时(122例)、日本(82例)、美国(79例)、中国(60例)和法国(50例)。
|
| 1:STL 1;2:SEDC; 3:ACG 2;4:Kniest; 5:ANFH+LCPD; 6:SEMD-S; 7:EO-OA; 8:PLSD-T; 9:EO-HM. 图 4 各疾病表型的男女患者例数 Fig. 4 Number of male and female patients with different disease phenotypes |
|
|
COL2A1基因突变导致的疾病通过常染色体显性遗传的方式在家族中遗传,该基因突变会导致骨骼、口腔颌面、听力系统和视力系统的异常表型,按照疾病严重程度和表型特征,对COL2A1基因突变导致的临床表型进行分类。见表 1。
| Severity | Phenotype | Abbr | OMIM Number |
| Lethal | Achondrogenesis, type Ⅱ or hypochondrogenesis | ACG 2 | 200610 |
| Platyspondylic skeletal dysplasia-Torrance type | PSLD-T | 151210 | |
| Severe | Dysspondyloen chondromatosis | DSC | None |
| Kniest dysplasia | Kniest | 156550 | |
| Multiple epiphyseal dysplasia | MED | 132450 | |
| Spondyloepiphyseal dysplasia(SED) congenita | SEDC | 183900 | |
| Spondyloepimetaphyseal dysplasia-Strudwick type | SEMD-S | 184250 | |
| Spondylometaphyseal dysplasia-Algerian type | SMD-A | 184253 | |
| Spondylometaphyseal dysplasia-Sutcliffe type | SMD-S | 184255 | |
| Spondyloperipheral dysplasia | SPD | 271700 | |
| Mild | Avascular necrosis of femoral head | ANFH | 608805 |
| Czech dysplasia | Czech | 609162 | |
| Early-onset high myopia | EO-HM | None | |
| Early-onset osteoarthritis | EO-OA | None | |
| Legg-calve-perthes disease | LCPD | 150600 | |
| Oto-spondylo-megaepiphyseal dysplasia | OS-MED | 215150 | |
| Spondyloepiphyseal dysplasia (SED) tarda | SED tarda | None | |
| Spondyloepiphyseal dysplasia (SED)-Stanescu type | SED-S | None | |
| Spondyloarthropathy | None | None | |
| Stickler syndrome, type Ⅰ | STL 1 | 108300 | |
| Wagner vitreoretinopathy | Wagner | 143200 |
该类表型共有2种,疾病特征为胎儿围产期夭折或出生后不久死亡。本研究结果显示:Ⅱ型软骨发育不全总计54例,患者通常会死于胎儿期或新生儿早期,该疾病的临床特征为不完全骨化、较短躯干、窄小胸部、膨隆腹部和短肢。研究[5]显示:Ⅱ型软骨发育不全病患者软骨细胞数量过多、软骨细胞体积过大且生长板紊乱。在54例突变中,比例最高为错义突变(50例),错义突变中48例为Gly突变。
扁平椎骨骼发育不良-Torrance分型总计12例,NISHIMMRA等[6]于1979年首次描述该疾病表型,该病为致死型表型,临床特征包括扁平椎、短肋骨、髂骨下端发育不全伴随较宽的坐骨和耻骨,或伴随短指及干骺端发育不良。该病12例突变中有10例突变发生在54号外显子,可推测COL2A1基因C端前肽区的突变与该疾病有较强的相关性[7-8]。
2.2.2 以脊柱病变为特征的表型该类表型特征为脊柱畸形,由于并发不同程度关节病变或视听系统的病变,因此细分为多个亚型。本研究结果显示:先天性脊柱骨骺发育不良总计88例,其临床特点是身材异常矮小、骨骺异常和扁平椎体,伴随症状有感觉神经性耳聋、高度近视、平脸、腭裂、小颌畸形、齿状突发育不全、髋内翻、膝外翻和马蹄足等。在88例突变中,比例最高为错义突变(81例),其中有60例位于Gly-X-Y重复序列的Gly位点,且p.Arg989Cys位点多次出现[9-10],可推断该位点是突变热点。
脊柱外周长骨末端发育不良-Strudwick分型总计18例,其临床表型特征:侏儒症、视力近视、视网膜脱落、腭裂、齿状突发育不良、胸壁前凸畸形(鸡胸)、脊柱侧弯、髋关节僵硬、髋内翻、膝外翻、马蹄内翻足和干骺端发育不良,在14例错义突变中,有13例为Gly突变。脊柱手指脚趾发育不良总计8例,ZHANG等[11]于1977年首次描述该疾病,其表型特征包括椎体异常融合、髋关节异常、较短掌骨和跖骨,手指及脚趾远端指(趾)骨较短,其中7例突变发生于C末端前肽区[12-13]。脊柱干骺端发育不良-Sutcliffe分型患者临床表型为轻度身材矮小、进展性髋内翻、轻度扁平椎、角骨折样损伤(特指长骨一端的角形部位骨折),突变位点分别为p.Gly345Asp和p.Gly945Ser突变[14]。脊柱骨骺发育不良-Stanescu分型患者的身高正常,伴发明显关节痛、进展性关节腔隙狭窄、扁平椎和髋内翻,有2例患者突变位点均位于p.Gly207Arg[15]。脊柱干骺端发育不良-Algerian分型患者临床特点是轻度身材矮小、严重膝外翻(特征性表型)、轻度扁平椎和干骺端异常,KOZLOWSKI等[16]在阿尔及利亚(Algerian)家系中发现该表型,2013年MATSUBAYASHI等[17]报道该疾病的突变位点位于p.Gly861Val。脊柱关节病临床特征仅有明显的关节病变和扁平椎,突变位点分别位于p.Arg519Cys和p.Arg1076Cys[18]。
2.2.3 以关节病变为特点的表型该类表型的特征为关节病变,由于伴发不同程度软骨发育不全或视听系统病变,因此细分为多个亚型。
研究[19]显示:Kniest发育不良总计报道了27例,该病临床特征:身材矮小、关节肿大、关节活动度下降、长骨呈哑铃型、胸呈铃形、椎体扁平,常伴腭裂、听力缺失、突眼和近视等症状。在27例突变中,有5例p.Ala302突变,可推测其为突变热点。多发性骨骺发育不良总计3例,临床特征:短肢侏儒症、关节疼痛和关节僵硬[20],该病主要由COMP等基因突变导致,COL2A1基因突变位点分别为p.Gly1179Arg和p.Gly1176Val[21],p.Gly678Arg突变可导致特征表型伴随双层髌骨[20]。
本研究包含股骨头缺血性坏死和Perthes病总计14例,包括5例Perthes病(儿童型股骨头坏死),CHEN等[22]于2004年在台湾家系中定位致病基因位于12号染色体,2005年LIU等[23]确认COL2A1基因突变位于p.Gly1170Ser位点,其临床特点:进展性股骨沟区疼痛和髋关节退化,近年还发现了3例p.Gly1170Ser突变[24-26];此外还有p.Gly717Ser、p.Gly582Ser和p.Ala184Thr这3个错义突变位点[23, 27-28]。研究[29-30]显示:Perthes病患者中存在p.Gly672Cys、p.Gly630Ser、p.Gly546Ser和p.Gly213Asp突变。
本研究包含早发型骨关节炎总计12例,其临床特征是关节疼痛、关节僵硬、关节腔隙狭窄、软骨下硬化或有骨赘,最终可导致关节功能丧失。早发性骨关节炎不如其他表型明显且多为伴发表型,但在少数病例中,该表型较为突出,COL2A1基因突变导致Ⅱ型胶原蛋白结构异常,使关节软骨弹性改变并最终导致骨关节炎[31],早发性骨关节炎多伴发轻度软骨发育不良。在11例错义突变中有8例Arg突变为Cys,可推断Arg位点与早发性骨关节炎有关联。
2.2.4 以眼部疾病为特征的表型该表型的特征为视觉系统退化和视网膜脱落,由于伴发不同程度的脊柱和关节发育不全,因此细分为多个亚型。
Stickler综合征Ⅰ型总计388例,是目前报道最多的表型。Stickler综合征包括6种亚型,Ⅰ~Ⅲ型为常染色体显性遗传,Ⅳ~Ⅵ型为常染色体隐形遗传[32]。Ⅰ型患者临床特征为玻璃体(膜)病变,可伴随视网膜脱落、面部畸形、腭裂和轻度脊柱骨骺发育异常[33-34]。本研究结果显示:在388例突变中比例最高是移码突变(141例),其次是无义突变(99例),Stickler综合征Ⅰ型中无义突变占COL2A1中无义突变的90%(99/110),可推测COL2A1无义突变与Stickler综合征Ⅰ型有关联。Wagner玻璃体视网膜病变的临床特征是早期白内障、中度近视和径向血管周围色素沉着(特征性视网膜表现)[35],突变位点分别为p.Cys57*和p.Gly267Asp[36-37]。早发型高度近视总计10例,高度近视的诊断标准是屈光度高于-0.6D,或轴向长度长于26 mm,高度近视是导致失明的重要病因。2015年SUN等[38]进行一项关于298例严重近视患者的研究发现:71例基因突变患者中有10例患者携带COL2A1基因突变。
2.2.5 特殊表型分类该类表型表现为非脊柱和非关节的特殊表型,因此单独分为一类。研究[39-42]显示:捷克发育不良患者总计7例,其表型为身高正常、第3和4脚趾较短(特殊表型)、进行性假性类风湿关节炎、扁平椎和脊柱关节病,目前已有5例患者的突变确认位于p.Arg275Cys位点。研究[43-44]显示:4例脊柱发育不良伴软骨瘤病患者特征表现为异常椎体、干骺端或长骨骨干处出现软骨瘤样损伤(特殊表型),已经发现4例儿童患者患有该病,突变位点分别是p.Gly753Asp、p.Gly600Asp、p.Gly1098Glu和p.Gly606Asp。耳-脊柱-大骨骺发育不良的临床表型主要为短肢、骨骺发育不良、面中部发育不良(鼻梁凹陷)和感觉神经性耳聋(特殊表型),同时常会并发腭裂及小颌畸形[45],2005年MIYAMOTO等[46]报道了1例发生于内含子的突变(c.709-2A>G)。
3 展望COL2A1基因突变导致一系列表型复杂的骨骼遗传疾病,目前已报道了600多例病例,本文作者发现数个突变热点,并推测一些潜在的基因型与表型的关系,旨在使临床医生更加深入了解COL2A1基因突变导致的遗传疾病表型,提高临床工作中对该遗传疾病的诊断率,并推动基因筛查在临床工作中的应用。在未来工作中还需要收集更多的病例报道和基础研究,致力于探讨COL2A1基因突变的具体致病机制以及明确的基因型与表型的关系。
| [1] |
TERHAL P A, VAN DOMMELEN P, LE MERRER M, et al. Mutation-based growth charts for sedc and other COL2A1 related dysplasias[J]. Am J Med Genet C Semin Med Genet, 2012, 160C(3): 205-216. DOI:10.1002/ajmg.c.31332 |
| [2] |
BARAT-HOUARI M, SARRABAY G, GATINOIS V, et al. Mutation update forCOL2A1 gene variants associated with type Ⅱ collagenopathies[J]. Hum Mutat, 2016, 37(1): 7-15. |
| [3] |
BRODSKY B, THIAGARAJAN G, MADHAN B, et al. Triple-helical peptides:An approach to collagen conformation, stability, and self-association[J]. Biopolymers, 2008, 89(5): 345-353. DOI:10.1002/bip.20958 |
| [4] |
ROUGHLEY P J. Articular cartilage and changes in arthritis:Noncollagenous proteins and proteoglycans in the extracellular matrix of cartilage[J]. Arthritis Res, 2001, 3(6): 342-347. DOI:10.1186/ar326 |
| [5] |
FAIVRE L, LE MERRER M, DOUVIER S, et al. Recurrence of achondrogenesis type Ⅱ within the same family:Evidence for germline mosaicism[J]. Am J Med Genet A, 2004, 126A(3): 308-312. DOI:10.1002/ajmg.a.20597 |
| [6] |
NISHIMURA G, NAKASHIMA E, MABUCHI A, et al. Identification of COL2A1 mutations in platyspondylic skeletal dysplasia, torrance type[J]. J Med Genet, 2004, 41(1): 75-79. DOI:10.1136/jmg.2003.013722 |
| [7] |
OKAMOTO T, NAGAYA K, ASAI H, et al. Platyspondylic lethal dysplasia torrance type with a heterozygous mutation in the triple helical domain of COL2A1 in two sibs from phenotypically normal parents[J]. Am J Med Genet A, 2012, 158A(8): 1953-1956. DOI:10.1002/ajmg.a.35509 |
| [8] |
DESIR J, CASSART M, DONNER C, et al. Spondyloperipheral dysplasia as the mosaic form of platyspondylic lethal skeletal dyplasia torrance type in mother and fetus with the same COL2A1 mutation[J]. Am J Med Genet A, 2012, 158A(8): 1948-1952. DOI:10.1002/ajmg.a.35301 |
| [9] |
ZHANG Z, HE J W, FU W Z, et al. Identification of three novel mutations in the COL2A1 gene in four unrelated chinese families with spondyloepiphyseal dysplasia congenita[J]. Biochem Biophys Res Co, 2011, 413(4): 504-508. DOI:10.1016/j.bbrc.2011.08.090 |
| [10] |
SILVEIRA K C, BONADIA L C, SUPERTI-FURGA A, et al. Six additional cases of SEDC due to the same and recurrent R989C mutation in the COL2A1 gene-the clinical and radiological follow-up[J]. Am J Med Genet A, 2015, 167A(4): 894-901. |
| [11] |
ZHANG Z, ZHAO S C, HE J W, et al. Identification of one novel mutation in the c-propeptide of COL2A1 in a chinese family with spondyloperipheral dysplasia[J]. Gene, 2013, 522(1): 107-110. DOI:10.1016/j.gene.2013.03.083 |
| [12] |
ZANKL A, ZABEL B, HILBERT K, et al. Spondyloperipheral dysplasia is caused by truncating mutations in the c-propeptide of COL2A1[J]. Am J Med Genet A, 2004, 129A(2): 144-148. DOI:10.1002/ajmg.a.30222 |
| [13] |
BEDESCHI M F, BIANCHI V, GENTILIN B, et al. Prenatal manifestation and management of a mother and child affected by spondyloperipheral dysplasia with a c-propeptide mutation in COL2A1:Case report[J]. Orphanet J Rare Dis, 2011, 6: 7. DOI:10.1186/1750-1172-6-7 |
| [14] |
MACHOL K, JAIN M, ALMANNAI M, et al. Corner fracture type spondylometaphyseal dysplasia:Overlap with type Ⅱ collagenopathies[J]. Am J Med Genet A, 2017, 173(3): 733-739. DOI:10.1002/ajmg.a.38059 |
| [15] |
JURGENS J, SOBREIRA N, MODAFF P, et al. Novel col2a1 variant (c.619g > a, p.Gly207arg) manifesting as a phenotype similar to progressive pseudorheumatoid dysplasia and spondyloepiphyseal dysplasia, stanescu type[J]. Hum Mutat, 2015, 36(10): 1004-1008. DOI:10.1002/humu.22839 |
| [16] |
KOZLOWSKI K, BACHA L, MASSEN R, et al. A new type of spondylo-metaphyseal dysplasia——algerian type. Report of five cases[J]. Pediatr Radiol, 1988, 18(3): 221-226. DOI:10.1007/BF02390399 |
| [17] |
MATSUBAYASHI S, IKEMA M, NINOMIYA Y, et al. COL2A1 mutation in spondylometaphyseal dysplasia algerian type[J]. Mol Syndromol, 2013, 4(3): 148-151. |
| [18] |
HOORNAERT K P, DEWINTER C, VEREECKE I, et al. The phenotypic spectrum in patients with arginine to cysteine mutations in the COL2A1 gene[J]. J Med Genet, 2006, 43(5): 406-413. |
| [19] |
MEREDITH S P, RICHARDS A J, BEARCROFT P, et al. Significant ocular findings are a feature of heritable bone dysplasias resulting from defects in type Ⅱ collagen[J]. Br J Ophthalmol, 2007, 91(9): 1148-1151. DOI:10.1136/bjo.2006.112482 |
| [20] |
DASA V, EASTWOOD J R B, PODGORSKI M, et al. Exome sequencing reveals a novel COL2A1 mutation implicated in multiple epiphyseal dysplasia[J]. Am J Med Genet A, 2019, 179(4): 534-541. DOI:10.1002/ajmg.a.61049 |
| [21] |
JACKSON G C, MITTAZ-CRETTOL L, TAYLOR J A, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia:A 7-year comprehensive analysis of the known disease genes identify novel and recurrent mutations and provides an accurate assessment of their relative contribution[J]. Hum Mutat, 2012, 33(1): 144-157. |
| [22] |
CHEN W M, LIU Y F, LIN M W, et al. Autosomal dominant avascular necrosis of femoral head in two taiwanese pedigrees and linkage to chromosome 12q13[J]. Am J Hum Genet, 2004, 75(2): 310-317. |
| [23] |
LIU Y F, CHEN W M, LIN Y F, et al. Type Ⅱ collagen gene variants and inherited osteonecrosis of the femoral head[J]. N Engl J Med, 2005, 352(22): 2294-2301. DOI:10.1056/NEJMoa042480 |
| [24] |
SU P Q, ZHANG L M, PENG Y, et al. A histological and ultrastructural study of femoral head cartilage in a new type Ⅱ collagenopathy[J]. Int Orthop, 2010, 34(8): 1333-1339. DOI:10.1007/s00264-010-0985-9 |
| [25] |
WANG L, PANH H, ZHU Z N. A genetic pedigree analysis to identify gene mutations involved in femoral head necrosis[J]. Mol Med Rep, 2014, 10(4): 1835-1838. DOI:10.3892/mmr.2014.2410 |
| [26] |
LIU F, XIONG Z Z, LIU Q, et al. COL2A1 mutation (c.3508G > A) leads to avascular necrosis of the femoral head in a chinese family:A case report[J]. Mol Med Rep, 2018, 18(1): 254-260. |
| [27] |
KISHIYA M, NAKAMURA Y, OHISHI H, et al. Identification of a novel COL2A1 mutation (c.1744G > A) in a japanese family:A case report[J]. J Med Case Rep, 2014, 8: 276. DOI:10.1186/1752-1947-8-276 |
| [28] |
SAKAMOTO Y, YAMAMOTO T, MIYAKE N, et al. Screening of the COL2A1 mutation in idiopathic osteonecrosis of the femoral head[J]. J Orthop Res, 2017, 35(4): 768-774. |
| [29] |
LI N, YU J, CAO X, et al. A novel p. Gly630ser mutation of COL2A1 in a chinese family with presentations of legg-calve-perthes disease or avascular necrosis of the femoral head[J]. PLoS One, 2014, 9(6): e100505. DOI:10.1371/journal.pone.0100505 |
| [30] |
KANNU P, IRVING M, AFTIMOS S, et al. Two novel COL2A1 mutations associated with a Legg-Calve-Perthes disease-like presentation[J]. Clin Orthop Relat Res, 2011, 469(6): 1785-1790. DOI:10.1007/s11999-011-1850-x |
| [31] |
RUKAVINA I, MORTIER G, VAN LAER L, et al. Mutation in the type Ⅱ collagen gene (col2ai) as a cause of primary osteoarthritis associated with mild spondyloepiphyseal involvement[J]. Semin Arthritis Rheum, 2014, 44(1): 101-104. DOI:10.1016/j.semarthrit.2014.03.003 |
| [32] |
SNEAD M P, YATES J R. Clinical and molecular genetics of stickler syndrome[J]. J Med Genet, 1999, 36(5): 353-359. |
| [33] |
WUBBEN T J, BRANHAM K H, BESIRLI C G, et al. Retinal detachment and infantile-onset glaucoma in stickler syndrome associated with known and novel COL2A1 mutations[J]. Ophthalmic Genet, 2018, 39(5): 615-618. DOI:10.1080/13816810.2018.1509355 |
| [34] |
ROCHA CABRERA P, CORDOVES DORTA L, SERRANO GARCIA M A, et al. Genetic variant of stickler's syndrome[J]. Arch Soc Esp Oftalmol, 2018, 93(3): 139-142. DOI:10.1016/j.oftal.2017.07.003 |
| [35] |
TRAN-VIET K N, SOLER V, QUIETTE V, et al. Mutation in collagen Ⅱ alpha 1 isoforms delineates Stickler and Wagner syndrome phenotypes[J]. Mol Vis, 2013, 19: 759-766. |
| [36] |
GUPTA S K, LEONARD B C, DAMJI K F, et al. A frame shift mutation in a tissue-specific alternatively spliced exon of collagen 2A1 in Wagner's vitreoretinal degeneration[J]. Am J Ophthalmol, 2002, 133(2): 203-210. |
| [37] |
KORKKO J, RITVANIEMI P, HAATAJA L, et al. Mutation in type Ⅱ procollagen (COL2A1) that substitutes aspartate for glycine alpha 1-67 and that causes cataracts and retinal detachment:Evidence for molecular heterogeneity in the wagner syndrome and the stickler syndrome (arthro-ophthalmopathy)[J]. Am J Hum Genet, 1993, 53(1): 55-61. |
| [38] |
SUN W M, HUANG L, XU Y, et al. Exome sequencing on 298 probands with early-onset high myopia:Approximately one-fourth show potential pathogenic mutations in retnet genes[J]. Invest Ophthalmol Vis Sci, 2015, 56(13): 8365-8372. DOI:10.1167/iovs.15-17555 |
| [39] |
HOORNAERT K P, MARIK I, KOZLOWSKI K, et al. Czech dysplasia metatarsal type:Another type Ⅱ collagen disorder[J]. Eur J Hum Genet, 2007, 15(12): 1269-1275. DOI:10.1038/sj.ejhg.5201913 |
| [40] |
BURRAGE L C, LU J T, LIU D S, et al. Early childhood presentation of czech dysplasia[J]. Clin Dysmorphol, 2013, 22(2): 76-80. DOI:10.1097/MCD.0b013e32835fff39 |
| [41] |
MATSUI Y, MICHIGAMI T, TACHIKAWA K, et al. Czech dysplasia occurring in a japanese family[J]. Am J Med Genet A, 2009, 149A(10): 2285-2289. DOI:10.1002/ajmg.a.33010 |
| [42] |
TZSCHACH A, TINSCHERT S, KAMINSKY E, et al. Czech dysplasia:Report of a large family and further delineation of the phenotype[J]. Am J Med Genet A, 2008, 146A(14): 1859-1864. DOI:10.1002/ajmg.a.32389 |
| [43] |
MERRICK B, CALDER A, WAKELING E. Dysspondyloenchondromatosis (DSC) associated with COL2A1 mutation:Clinical and radiological overlap with spondyloepimetaphyseal dysplasia-strudwick type (SEMD-S)[J]. Am J Med Genet A, 2015, 167A(12): 3103-3107. |
| [44] |
GUNES N, YESIL G, BENG K, et al. Longitudinal follow-up of two patients with dysspondyloenchondromatosis due to novel heterozygous mutations in COL2A1[J]. Mol Syndromol, 2018, 9(3): 134-140. DOI:10.1159/000488438 |
| [45] |
TOKGOZ-YILMAZ S, SAHLI S, FITOZ S, et al. Audiological findings in otospondylomegaepiphyseal dysplasia (OSMED) associated with a novel mutation in COL2A1[J]. Int J Pediatr Otorhinolaryngol, 2011, 75(3): 433-437. DOI:10.1016/j.ijporl.2010.12.004 |
| [46] |
MIYAMOTO Y, NAKASHIMA E, HIRAOKA H, et al. A type Ⅱ collagen mutation also results in oto-spondylo-megaepiphyseal dysplasia[J]. Hum Genet, 2005, 118(2): 175-178. |
 2020, Vol. 46
2020, Vol. 46


