扩展功能
文章信息
- 闫圣玉, 谢亚锋, 许志杰, 刘英, 丁雅婷, 张侨, 刘菀莹, 刘丽兵
- YAN Shengyu, XIE Yafeng, XU Zhijie, LIU Ying, DING Yating, ZHANG Qiao, LIU Wanying, LIU Libing
- 抗菌肽LL-37对结肠癌小鼠肿瘤生长的抑制作用及其机制
- Inhibitory effect of antimicrobial peptide LL-37 on tumorgrowth of mice with colon cancer and its mechanism
- 吉林大学学报(医学版), 2020, 46(03): 575-581
- Journal of Jilin University (Medicine Edition), 2020, 46(03): 575-581
- 10.13481/j.1671-587x.20200324
-
文章历史
- 收稿日期: 2019-08-06
2. 南华大学附属第二医院重症医学科, 湖南 衡阳 421001;
3. 南华大学附属南华医院肛肠科, 湖南 衡阳 421002
2. Department of Critical Medicaine, Second Affiliated Hospital, Nanhua University, Hengyang 421001, China;
3. Department of Anorectal Surgery, Affiliated Nanhua Hospital, Nanhua University, Hengyang 421002, China
抗菌肽LL-37作为天然的免疫分子,具有广谱杀菌和抗肿瘤活性[1]。研究[2]显示:LL-37表达水平与肠道微生物的种类和数量有密切关联。肠道微生态失衡是影响结肠癌发生发展的重要原因[3],因此LL-37表达异常可能与结肠癌的发生发展有关联。另有研究[4-6]显示:LL-37能抑制胃癌、急性髓样细胞和淋巴细胞性白血病等恶性肿瘤的发生发展。研究[7]显示:LL-37在正常结肠黏膜组织中高表达,但在结肠癌组织中低表达,并且外源LL-37处理可诱导结肠癌细胞凋亡,因而推测LL-37在结肠癌发生发展过程中起抑癌基因的作用,但目前关于LL-37抑制结肠癌发生发展的具体机制尚未见报道。本研究通过制备结肠癌小鼠模型,观察LL-37过表达对结肠癌荷瘤小鼠肿瘤生长情况和细胞凋亡水平的影响,进一步探讨LL-37在结肠癌中的作用机制,以期为寻找人结肠癌病因和预防提供新思路,为临床上肿瘤治疗提供新的潜在靶点。
1 材料与方法 1.1 细胞、实验动物、主要试剂和仪器人结肠癌HT-29细胞株(BNCC)购自北京北纳生物公司。清洁级6~8周龄BALB/c小鼠,体质量20~22g,购自湖南远泰生物技术有限公司,动物使用许可证号:SYXK(湘)2016-0004;分笼饲养,饲养温度为19℃~23℃,相对湿度为30%~70%,光照周期为12 h/12 h,自由饮水摄食。LL37 cDNA质粒购自美国Invitrogen公司,磷酸腺苷活化的蛋白激酶(AMPK)抑制剂Dorsomorphin(Dor,50 μmol·L-1)购自美国Sigma-Aldrich公司,逆转录试剂盒PrimeScriptTM RT Reagent Kit和荧光定量试剂盒TB Green® Premix Ex TaqTM Ⅱ均购自日本TaKaRa公司,GV314腺病毒载体购自上海吉凯生物公司,一抗鼠抗LL-37购自美国Santa Cruz公司,一抗兔抗p-AMPK(1:1 000)、兔抗p-mTOR(1:1 000)、兔抗LC3A(1:50 000)、兔抗LC3B(1:1 000)、兔抗GAPDH(1:2 500)、兔抗AMPK(1:1 000),兔抗mTOR(1:2 000)、兔抗Beclin1(1:2 000)、兔抗Atg5(1:500)和二抗IgG(1:2 000)均购自英国Abcam公司。倒置显微镜(TE2000-U)购自日本尼康公司,SDS-PAGE蛋白电泳仪购自美国Bio-Rad公司,实时荧光定量PCR仪(7500)购自美国ABI公司。
1.2 LL-37过表达结肠癌HT-29细胞的构建和细胞中LL-37 mRNA和蛋白表达水平的检测从LL-37质粒中扩增出目的基因,扩增引物序列:Forward 5′-AGGTCGACTCTAGAGGATCCCGCCACCATGAAGACCCAAAGGGATG -3′,Reverse 5′-TCCTTGTAGTCCATACCGGACTCTGTCCTGGGTACAAG-3′。再将GV314腺病毒载体进行双酶切反应(Bam HⅠ和AgeⅠ),采用T4连接酶将目的基因插入载体中,构建pGV314-LL-37重组腺病毒载体。将pGV314-LL-37腺病毒包装、扩增并进行滴度测定。按LipofectamineTM2000试剂盒说明书将重组腺病毒载体转染至HEK293细胞中,扩增后检测腺病毒滴度。当HT-29细胞达到70%融合时,按感染复数(MOI)值为75采用腺病毒感染HT-29细胞48 h后,加入嘌呤霉素(1.5 mg·L-1)筛选稳定转染细胞,并采用荧光显微镜观察感染效率,收集细胞,结肠癌HT-29细胞中LL-37 mRNA表达水平采用2-ΔΔCt法计算,LL-37蛋白表达水平=LL-37蛋白条带灰度值/GAPDH条带灰度值。
1.3 实验动物分组和结肠癌HT-29荷瘤小鼠模型的制备在适应性饲养1周后,将BALB/c小鼠随机分为5组:对照组,接种未经感染的HT-29细胞;空载组,接种感染空载质粒的HT-29细胞;LL-37过表达组,接种感染LL-37过表达载体的HT-29细胞;AMPK抑制剂组,接种感染空载体的HT-29细胞后,立刻尾静脉注射2 mg·kg-1 Dox,每日1次,持续24 d[8];联合作用组,接种感染LL-37过表达载体的HT-29细胞后,立刻尾静脉注射2 mg·kg-1 Dox,每日1次,持续24 d。每组6只小鼠。将HT-29细胞制备成单细胞悬液,调整细胞浓度为2×107mL-1,采用一次性无菌注射器在各组裸小鼠右侧腋下行皮下注射,每只裸小鼠注射剂量为0.1 mL。
1.4 各组小鼠肿瘤生长情况检测待完成小鼠移植瘤造模,每日观察皮下肿瘤的生长情况,每4 d采用游标卡尺测量肿瘤的长径(a)和短径(b),计算肿瘤体积(V):V(cm3)=a×b2/2,并绘制肿瘤生长曲线。24 d后采用断颈法处死小鼠,剥离移植瘤后称质量,取平均值(g),计算各组小鼠抑瘤率。抑瘤率=(对照组平均肿瘤质量-实验组平均肿瘤质量)/对照组平均肿瘤质量×100%。
1.5 TUNEL染色检测各组小鼠细胞凋亡情况根据TUNEL试剂盒操作步骤进行实验。先制作移植瘤组织石蜡切片,再将切片在60℃下烘烤15 min,二甲苯脱蜡,梯度乙醇脱水。然后用蛋白酶K在室温下处理10 min,加入制备好的TUNEL反应工作液,37℃下孵育1 h。采用3%H2O2甲醇溶液室温处理10 min。在37℃下加入过氧化物酶溶液孵育30 min。随后,采用二氨基联苯胺(DAB)对切片进行染色。苏木精复染后,切片采用梯度乙醇脱水,二甲苯透明,树脂封闭。最后在显微镜下观察TUNEL阳性细胞,即凋亡细胞,显微镜下观察显示TUNEL阳性细胞呈黄褐色。
1.6 qRT-PCR法检测各组小鼠肿瘤组织中LL-37 mRNA表达水平采用TRIzol试剂从移植瘤组织中提取分离总RNA。根据逆转录cDNA试剂盒说明书进行cDNA链合成。LL-37 mRNA引物序列:Forward 5′-CCACCATGGGCCTGGTGATGCCT- CTGGCCATC-3′,Reverse 5′-TGTACACTAGGACTCTGTCCTGGGTACAAG-3′;GAPDH为内参,Forward 5′-TCAACAGCAACTCCCACTCTTCCA-3′,Reverse 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′。定量分析反应体系为25 μL:12.5 μL 2×SYBR® Premix Ex TaqTM Ⅱ、1 μL正向和反向引物、2 μL cDNA模板和8.5 μL ddH2O。采用2-ΔΔCt法分析各组小鼠肿瘤组织中LL-37 mRNA表达水平。
1.7 Western blotting法检测各组小鼠肿瘤组织中自噬相关蛋白Beclin1、Atg5和LC3Ⅰ及LC3Ⅱ和AMPK/mTOR通路相关蛋白AMPK、p-AMPK、mTOR和p-mTOR表达水平取各组小鼠移植瘤组织,剪成小块置于EP管中,再加入含预冷的RIPA裂解液和磷酸酶抑制剂的缓冲液进行匀浆。提取出总蛋白后,置于95℃中震荡5 min使蛋白变性,将20 μg样品在10%聚丙烯酰胺凝胶上分离,并转移到硝酸纤维素膜,采用5%脱脂奶粉封闭,然后与对应的一抗抗体LC3Ⅰ、LC3Ⅱ、Atg5、Beclin1、p-AMPK、AMPK、p-mTOR和mTOR于4℃孵育过夜。GAPDH作为内参。随后采用1×Tris缓冲盐水和Tween-20(TBST)洗涤3次,每次10 min。加入辣根过氧化物酶标记的二抗室温孵育1 h,采用TBST清洗3次,每次10 min。加入增强化学发光剂ECL,曝光显影后室温晾干,分析蛋白条带灰度值,计算目的蛋白表达水平。目的蛋白表达水平=目的蛋白条带灰度值/ GAPDH条带灰度值。
1.8 统计学分析采用SPSS19.0统计软件进行统计学分析。各组HT-29细胞中LL-37 mRNA和蛋白表达水平,小鼠移植瘤平均质量和体积,各组小鼠肿瘤组织中自噬相关蛋白Beclin1、Atg5、LC3Ⅱ及LC3Ⅱ和AMPK/mTOR通路相关蛋白AMPK、p-AMPK、mTOR及p-mTOR表达水平以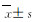
荧光显微镜下观察到pGV314-LL-37感染结肠癌HT-29细胞48 h后效率超过80%。与对照组和空载组比较,LL-37过表达组HT-29细胞中LL-37 mRNA和蛋白表达水平升高(P < 0.01)。见图 1和图 2(插页八)。

|
| A: Electrophoregram of expressions of LL-37 protein in HT-29 cells in various groups deteceted by Western blotting method (Lane 1: Control group; Lane 2: Empty vector group; Lane 3: LL-37 over-expression group); B: Expression levels of LL-37 mRNA detected by qRT-PCR method(1:Control group; 2:Empty vector group; 3:LL-37 over-expression group); C:Expression levels of LL-37 protein(1:Control group; 2:Empty vector group; 3:LL-37 over-expression group).* P < 0.01 vs control group; △ P < 0.01 vs empty vector group. 图 1 感染48h各组结肠癌HT-29细胞中LL-37 mRNA和蛋白表达水平 Fig. 1 Expression levels of LL-37 mRNA and protein in colon cancer HT-29 cells in various groups 48 hafter infection |
|
|
|
| 图 2 对照组(A)和空载组(B)pGV314-LL-37感染结肠癌HT-29细胞情况 Fig. 2 Situation of human colon cancer HT-29 cells infected by pGV314-LL-37 in control group(A) and empty vector group(B) |
|
|
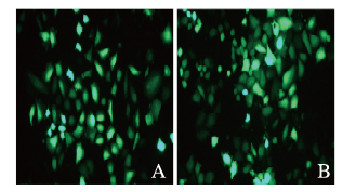
HT-29荷瘤小鼠肿瘤生长的检测指标包括感染24 d后肿瘤质量和抑瘤率。与对照组和空载组比较,LL-37过表达组小鼠肿瘤质量和体积明显降低(P < 0.01),抑瘤率升高(P < 0.05);AMPK抑制剂组小鼠肿瘤质量和体积明显升高(P < 0.05或P < 0.01),抑瘤率降低(P < 0.05);与LL-37过表达组比较,联合组小鼠肿瘤质量和体积明显升高(P < 0.05或P < 0.01),抑瘤率降低(P < 0.05)。见表 1和图 3。
(n=6,  | |||||||||||||||||||||||||||||
| Group | Weight of tumor(m/g) | Volume of tumor(V/cm3) | Inhibitory rate of tumor(η/%) | ||||||||||||||||||||||||||
| Control | 2.42±0.67 | 2.57±0.22 | - | ||||||||||||||||||||||||||
| Empty vector | 2.27±0.52 | 2.31±0.34 | 6.19 | ||||||||||||||||||||||||||
| LL-37 over-expression | 0.85±0.41**△△ | 1.15±0.23**△△ | 69.01△ | ||||||||||||||||||||||||||
| AMPK inhibitor | 2.91±0.22*△ | 3.18±0.38**△△ | -20.25△ | ||||||||||||||||||||||||||
| Combination | 1.54±0.55# | 1.77±0.20## | 36.36# | ||||||||||||||||||||||||||
| F | 15.82 | 44.79 | - | ||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | - | ||||||||||||||||||||||||||
| “-”:No data. * P < 0.05, * * P < 0.01 vs control group; △ P < 0.05, △△ P < 0.01 vs empty vector group; # P < 0.05, ## P < 0.01 vs LL-37 over-expression group. | |||||||||||||||||||||||||||||
|
| 图 3 感染不同时间各组小鼠肿瘤体积 Fig. 3 Tumor volumes of mice in various groups at different time after infection |
|
|
与对照组和空载组比较,LL-37过表达组小鼠肿瘤组织中黄褐色凋亡细胞明显增多,AMPK抑制剂组小鼠肿瘤组织中凋亡细胞数减少;与LL-37过表达比较,联合组小鼠肿瘤组织中细胞凋亡数减少。见图 4(插页八)。
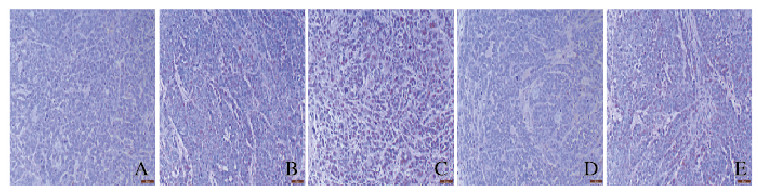
|
| A: Control group; B: Empty vector group; C: LL-37 over-expression group; D: AMPK inhibitor group; E: Combination group 图 4 TUNEL法观察各组小鼠肿瘤细胞凋亡形态表现(Bar=50 μm) Fig. 4 Apoptotic morphology of tumor cells of mice in various groups observed by TUNEL method (Bar=50 μm) |
|
|
与对照组和空载组比较,LL-37过表达组小鼠肿瘤组织中自噬相关蛋白Beclin1和Atg5表达水平及LC3Ⅱ/LC3Ⅰ比值均明显升高(P < 0.01);AMPK抑制剂组小鼠肿瘤组织中自噬相关蛋白Beclin1和Atg5表达水平及LC3Ⅱ/LC3Ⅰ比值明显降低(P < 0.05或P < 0.01)。与LL-37过表达组比较,联合组小鼠肿瘤组织中自噬相关蛋白Beclin1和Atg5表达水平及LC3Ⅱ/LC3Ⅰ比值明显降低(P < 0.05或P < 0.01)。见图 5。

|
| A: Electrophoregram of expressions of LC3Ⅰ, LC3Ⅱ, Atg5 and Beclin1 proteins deteceted by Western blotting method (Lane 1: Control group; Lane 2: Empty vector group; Lane 3: LL-37 over-expression group; Lane 4: AMPK inhibitor group; Lane 5: Combination group); B: Ratios of LC3Ⅱ/LC3Ⅰ(1:Control group; 2:Empty vector group; 3:LL-37 over-expression group; 4:AMPK inhibitor group; 5:Combination group); C:Expression level of Atg5 protein (1:Control group; 2:Empty vector group; 3:LL-37 over-expression group; 4:AMPK inhibitor group; 5:Combination group); D: Expression level of Beclin1 protein(1:Control group; 2:Empty vector group; 3:LL-37 over-expression group; 4:AMPK inhibitor group; 5:Combination group).*P < 0.01 vs control group; △ P < 0.05, △△P < 0.01 vs empty vector group; # P < 0.05 vs LL-37 over-expression group. 图 5 Western blotting法检测各组小鼠肿瘤组织中自噬相关蛋白表达 Fig. 5 Expressions of autophagy-related proteins in tumor tissue of mice in various groups detected by Western blotting assay |
|
|
与对照组和空载组比较,LL-37过表达组小鼠肿瘤组织中p-AMPK/AMPK比值明显升高(P < 0.01),p-mTOR/mTOR比值明显降低(P < 0.01);AMPK抑制剂组小鼠肿瘤组织中p-AMPK/AMPK比值明显降低(P < 0.05),p-mTOR/mTOR比值明显升高(P < 0.01)。与LL-37过表达组比较,联合组小鼠肿瘤组织中p-AMPK/AMPK比值明显降低(P < 0.05),p-mTOR/mTOR比值明显升高(P < 0.01)。见图 6。

|
| A: Electrophoregram of expressions of p-AMPK, AMPK, p-mTOR and mTOR deteceted by Western blotting method(Lane 1: Control group; Lane 2: Empty vector group; Lane 3: LL-37 over-expression group; Lane 4: AMPK inhibitor group; Lane 5: Combination group); B: Ratios of p-AMPK/AMPK(1:Control group; 2:Empty vector group; 3:LL-37 over-expression group; 4:AMPK inhibitor group; 5:Combination group); C: Ratio of p-mTOR/mTOR(1:Control group; 2:Empty vector group; 3:LL-37 over-expression group; 4:AMPK inhibitor group; 5:Combination group).*P < 0.01 vs control group; △ P < 0.05, △△P < 0.01 vs empty vector group; #P < 0.05, ##P < 0.01 vs LL-37 over-expression group. 图 6 Western blotting法检测各组小鼠肿瘤组织中AMPK/mTOR信号通路蛋白表达 Fig. 6 Expressions of AMPK/mTOR signaling pathway-related proteins in tumor tissue of mice in various groups detected by Western blotting method |
|
|
抗菌肽是一类具有抗菌活性的碱性多肽物质,能通过破坏细胞膜的功能杀死细菌,并干扰细胞壁的合成[9]。组织蛋白酶是抗菌肽的一大家族,其中LL-37是现阶段发现的唯一一种人类组织蛋白酶抑制素类抗菌肽,由人类抗菌蛋白HCAP-18的C-端部分剪切形成[10]。临床研究[11-12]证明:在急性腹泻和淋病奈瑟菌感染期间,黏膜上皮表面的LL-37表达下调。丁酸盐(一种来自结肠中膳食纤维的细菌发酵产物)可以抑制志贺氏菌病家兔模型中组织蛋白酶表达的降低[13]。-4-苯基丁酸盐(PBA)可以在mRNA和蛋白水平上诱导活性LL-37表达[14]。临床研究[15]显示:健康个体口服PBA后,能在人单核细胞源巨噬细胞中诱导LL-37表达。YUK等[16]研究表明:LL-37能激活人巨噬细胞的自噬,从而降低结合分歧杆菌的细胞内存活率。有研究[17]显示:PBA诱导的自噬依赖于LL-37,并且经受体介导,上述研究结果提示LL-37参与了细胞自噬过程。
研究[18]显示:LL-37在肠癌组织中呈异常低表达,而在正常组织中呈高表达,并且LL-37对肠癌的影响与肠道微生物菌群种类有关联。但LL-37在结肠癌中的作用机制尚未明确。本研究通过构建过表达LL-37腺病毒载体,感染人结肠癌HT-29细胞,经荧光观察、qRT-PCR和Western blotting法检测肠癌组织中LL-37表达,说明成功得到稳定过表达LL-37的人结肠癌HT-29细胞。本研究观察小鼠移植瘤生长情况(质量、体积和抑瘤率)、肿瘤细胞凋亡、自噬和AMPK/mTOR信号通路蛋白表达水平,结果显示:过表达LL-37能明显抑制人结肠癌HT-29移植瘤的生长,降低移植瘤的质量和体积,提高抑瘤率,而且还能明显促进细胞凋亡,说明LL-37对人结肠癌HT-29移植瘤具有抑制作用。其次,自噬作为细胞的核心功能之一,参与了癌细胞凋亡[19]。本研究检测自噬相关蛋白表达水平,结果表明:LL-37能明显提高人结肠癌HT-29移植瘤的自噬水平,说明LL-37参与了人结肠癌细胞自噬过程,这与之前的研究[7]结果一致。研究[19-21]表明:AMPK激活可能通过mTOR负调节导致自身吞噬,活化的AMPK通过磷酸化结节性硬化复合体2(TSC2)抑制TORC1的活性。AMPK可直接磷酸化UNC-51样激酶1(ULK1)诱导自噬[22-23]。本研究结果表明:低表达AMPK能明显促进移植瘤生长,提高肿瘤质量和体积,抑制移植瘤细胞凋亡,并且降低移植瘤自噬水平。低表达AMPK还能逆转过表达LL-37对移植瘤生长、凋亡和自噬作用,表明AMPK/mTOR信号通路参与了LL-37对人结肠癌HT-29移植瘤的抑制作用。
综上所述,LL-37通过AMPK/mTOR信号通路影响人结肠癌HT-29移植瘤的生长、细胞凋亡和自噬水平。
| [1] |
ASHBY M, PETKOVA A, GANI J, et al. Use of peptide libraries for identification and optimization of novel antimicrobial peptides[J]. Curr Top Med Chem, 2017, 17(5): 537-553. |
| [2] |
WANG S, ZENG XF, YANG Q, et al. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry[J]. Int J Mol Sci, 2016, 17(5): E603. |
| [3] |
CIPE G, IDIZ U O, FIRAT D, et al. Relationship between intestinal microbiota and colorectal cancer[J]. World J Gastrointest Oncol, 2015, 7(10): 233-240. |
| [4] |
PIKTEL E, NIEMIROWICZ K, WNOROWSKA U, et al. The role of cathelicidin LL-37 in cancer development[J]. Arch Immunol Ther Exp (Warsz), 2016, 64(1): 33-46. |
| [5] |
OKAMOTO R, GERY S, KUWAYAMA Y, et al. Novel Gemini vitamin D3 analogs:large structure/function analysis and ability to induce antimicrobial peptide[J]. Int J Cancer, 2014, 134(1): 207-217. |
| [6] |
YANG Y H, ZHENG G G, LI G, et al. Expression of LL-37/hCAP-18 gene in human leukemia cells[J]. Leuk Res, 2003, 27(10): 947-950. |
| [7] |
REN S X, CHENG A S, TO K F, et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer[J]. Cancer Res, 2012, 72(24): 6512-6523. |
| [8] |
KIM K H, LEE I S, PARK J Y, et al. Cucurbitacin B induces hypoglycemic effect in diabetic mice by regulation of AMP-activated protein kinase alpha and glucagon-like peptide-1 via bitter taste receptor signaling[J]. Front Pharmacol, 2018, 9: 1071. DOI:10.3389/fphar.2018.01071 |
| [9] |
KANG H K, KIM C, SEO C H, et al. The therapeutic applications of antimicrobial peptides (AMPs):a patent review[J]. J Microbiol, 2017, 55(1): 1-12. |
| [10] |
JI P, ZHOU Y X, YANG Y B, et al. Myeloid cell-derived LL-37 promotes lung cancer growth by activating Wnt/β-catenin signaling[J]. Theranostics, 2019, 9(8): 2209-2223. |
| [11] |
ISLAM D, BANDHOLTZ L, NILSSON J, et al. Downregulation of bactericidal peptides in enteric infections:a novel immune escape mechanism with bacterial DNA as a potential regulator[J]. Nat Med, 2001, 7(2): 180-185. |
| [12] |
BERGMAN P, JOHANSSON L, ASP V, et al. Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37[J]. Cell Microbiol, 2005, 7(7): 1009-1017. |
| [13] |
RAQIB R, SARKER P, BERGMAN P, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic[J]. Proc Natl Acad Sci U S A, 2006, 103(24): 9178-9183. |
| [14] |
STEINMANN J, HALLDÓRSSON S, AGERBERTH B, et al. Phenylbutyrate induces antimicrobial peptide expression[J]. Antimicrob Agents Chemother, 2009, 53(12): 5127-5133. |
| [15] |
MILY A, REKHA R S, KAMAL S M, et al. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages:a dose finding study for treatment of tuberculosis[J]. BMC Pulm Med, 2013, 13: 23. DOI:10.1186/1471-2466-13-23 |
| [16] |
YUK J M, SHIN D M, LEE H M, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin[J]. Cell Host Microbe, 2009, 6(3): 231-243. |
| [17] |
REKHA R S, RAO MUVVA S S, WAN M, et al. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages[J]. Autophagy, 2015, 11(9): 1688-1699. |
| [18] |
高旭峰, 程先硕, 周坦, 等. 抗菌肽LL-37在结直肠癌中的研究进展[J]. 现代肿瘤医学, 2018, 26(9): 1441-1444. |
| [19] |
PIERZYNOWSKA K, GAFFKE L, PODLACHA M, et al. Mucopolysaccharidosis and autophagy: controversies on the contribution of the process to the pathogenesis and possible therapeutic applications[J/OL]. Neuromolecular Med, 2019. https://doi.org/10.1007/s12017-019-08559-1.
|
| [20] |
WANG Q, WEI S, ZHOU S, et al. Hyperglycemia aggravates acute liver injury by promoting liver-resident macrophage NLRP3 inflammasome activation via the inhibition of AMPK/mTOR-mediated autophagy induction[J/OL]. Immunol Cell Biol, 2020, 98(1): 54-66.
|
| [21] |
RANEK M J, KOKKONEN-SIMON K M, CHEN A N, et al. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress[J]. Nature, 2019, 566(7743): 264-269. |
| [22] |
DITE T A, LING N X Y, SCOTT J W, et al. The autophagy initiator ULK1 sensitizes AMPK to allosteric drugs[J]. Nat Commun, 2017, 8(1): 571. |
| [23] |
刘帛岩, 李松岩, 张红亮, 等. 肿瘤细胞减灭术联合腹腔热灌注化疗对结肠癌与直肠癌腹膜转移的远期疗效比较[J]. 解放军医学杂志, 2020, 45(1): 79-83. |
 2020, Vol. 46
2020, Vol. 46


