扩展功能
文章信息
- 石光环, 周世平, 徐东升, 王琇
- SHI Guanghuan, ZHOU Shiping, XU Dongsheng, WANG Xiu
- 扩增的NK细胞对胃癌细胞的杀伤作用及其机制
- Killing effect of amplified NK cells on gastric cancer cells and its mechanism
- 吉林大学学报(医学版), 2020, 46(03): 530-535
- Journal of Jilin University (Medicine Edition), 2020, 46(03): 530-535
- 10.13481/j.1671-587x.20200317
-
文章历史
- 收稿日期: 2019-04-15
2. 吉林大学第一医院肿瘤科, 吉林 长春 130021
2. Department of Oncology, First Hospital, Jilin University, Changchun 130021, China
自然杀伤(natural killer,NK)细胞是人体天然免疫系统的重要组成细胞,其能够杀伤肿瘤细胞[1-2]。NK细胞治疗对血液肿瘤有很好疗效[3],对肺癌[4]和肝癌[5]也有一定临床疗效。研究[6]表明:胃癌患者外周血中NK细胞数明显低于正常人,且NK细胞数与胃癌患者的临床病理分期有密切关联,NK细胞数越少,胃癌患者的临床病理分期越晚。胃癌患者NK细胞活化程度也明显低于正常人[7]。有研究[8]显示:如果能将胃癌患者外周血中NK细胞大量扩增激活,再回输患者,就有可能增加胃癌患者NK细胞数和改善其功能缺陷,提高胃癌患者的临床治疗效果。本研究旨在探讨扩增后NK细胞对胃癌细胞的杀伤作用及其机制,以期为NK细胞治疗胃癌提供理论依据。
1 材料与方法 1.1 细胞、主要试剂和仪器SGC7901细胞购自北京北纳创联生物技术研究院,SGC7901细胞用含有10%胎牛血清(fetal bovine serum,FBS)的RPMI-1640培养基培养传代,每2~3 d换液1次。FBS和RPMI-1640培养基购自美国Gibco公司,赫赛汀购自瑞士罗氏公司,重组人白细胞介素2(interleukin-2,IL-2)和重组人白细胞介素5(interleukin-5,IL-5)购自德国美天旎生物技术有限公司,X-VIVO培养基购自瑞士Lonza公司,淋巴细胞分离液购自挪威Nycomed Pharma AS公司,鼠抗人CD3-APC、CD56-PE、KIR2DL1-PE、KIR3DL1-APC、NKG2D-APC和DNAM-1-PE单克隆抗体购自美国BD Biosciences公司,人NK细胞富集试剂盒购自美国Stemcell Technologies公司,钙黄绿素购自日本同仁化学研究所。倒置荧光显微镜购自日本Olympus公司,CO2培养箱购自美国Thermo公司,离心机购自德国Beckman公司,流式细胞仪购自美国BD公司,酶标仪购自美国BioTeck公司。
1.2 外周血单个核细胞(peripheral blood mononuclear cells,PBMCs)分离收集15例胃癌患者的临床资料,其中男性8例,女性15例,年龄36~75岁,中位年龄58岁。根据TNM分期分为Ⅰ期7例,Ⅱ期5例,Ⅲ期3例。15例胃癌患者外周血经3 000 r·min-1离心10 min,将血浆和血细胞分开,血浆置于56 ℃恒温培养箱中30 min以灭活补体,血细胞采用淋巴细胞分离液分离PBMCs,并采用生理盐水洗涤2次。
1.3 NK细胞体外扩增采用含有5%自体血浆、50 μg·L-1赫赛汀、500 U·mL-1重组人IL-2和10 μg·L-1重组人IL-15的X-VIVO完全培养基将PBMCs密度调整至3×106 mL-1,接种于25 cm2培养瓶中,标记为培养第0天,并置于37 ℃、5% CO2恒温培养箱中培养,每2~3 d半量更换1次X-VIVO培养基(含有5%自体血浆,500 U·mL-1重组人IL-2和10 μg·L-1重组人IL-15)。培养至第14天,收集细胞,开始后续实验。
1.4 NK细胞的形态表现观察和NK细胞百分比在培养的第0、2、4、6、8、10、12和14天观察NK细胞的形态,记录培养液体积,取0.5 mL NK细胞培养基,进行细胞计数,将细胞密度调整为1×106 mL-1,取100 μL细胞加入至流式管中,加入CD3-APC和CD56-PE单克隆抗体,室温下避光孵育15 min,PBS洗涤2次,100 μL PBS悬起细胞,采用流式细胞术检测NK细胞百分比。按照0.5 mL培养基中细胞数计算培养基中细胞总数,并根据流式细胞术检测的NK细胞百分比计算NK细胞实际数。
1.5 NK细胞分离采用人NK细胞富集试剂盒分离PBMCs中的NK细胞和扩增培养后的NK细胞。
1.6 细胞分组和杀伤作用检测将细胞按照靶细胞和效应细胞比例不同分为实验组、最小释放组和最大释放组。靶细胞处理:收集培养的SGC7901细胞,以PBS洗涤1次,并采用PBS将其密度调整至1×106 mL-1,取1 mL细胞,加入钙黄绿素,使其终浓度为1 μmol·L-1,置于37 ℃、5%CO2培养箱中孵育30 min。PBS洗涤2次后,采用含5% FBS的RPMI-1640培养基重悬细胞,并将细胞密度调整至5×104 mL-1。效应细胞处理:将纯化的NK细胞采用含有5% FBS的RPMI-1640培养基重悬细胞,并将其密度调整至5×105 mL-1(5:1)。实验组:加入100 μL靶细胞和效应细胞,加入圆底无菌带盖96孔板中;最小释放组:加入100 μL靶细胞和100 μL PBS;最大释放组:100 μL靶细胞和100 μL含有10%TritonX-100的PBS;每组3个复孔。在37℃、5%CO2培养箱中避光孵育4 h。从每孔中轻轻吸取100 μL上清置于新的平底96孔平板中。采用酶标仪检测上清的荧光值(激发光波长为485 nm,发射光波长为528 nm),计算扩增前后NK细胞对肿瘤细胞的杀伤作用。杀伤活性=(实验组荧光值-最小释放组荧光值)/(最大释放组荧光值-最小释放组荧光值)×100%。
1.7 扩增前后NK细胞表面活化性受体表达和表面抑制性受体表达收集细胞,以PBS洗涤1次,并采用PBS将其密度调整至1×106 mL-1,取100 μL细胞加入流试管中,加入CD3-PerCP和CD56-FITC单克隆抗体,同时分别加入KIR2DL1-PE、KIR3DL1-APC、NKG2D-APC和DNAM-1-PE单克隆抗体及其同型对照抗体,避光孵育15 min后,PBS洗涤1次,100 μL PBS重悬细胞,采用流式细胞术进行检测。采用FlowJo Version 10软件分析流式细胞术检测结果进行表型分析。
1.8 统计学分析采用GraphPad Prism Version5.01软件分析数据,扩增前后NK细胞百分比、NK细胞对胃癌细胞的杀伤活性、NK细胞表面活化性受体表达和表面抑制性受体表达百分比均以x ±s表示,组间比较采用配对t检验。以P < 0.05表示差异有统计学意义。
2 结果 2.1 扩增前后NK细胞形态表现培养第0天PBMCs中NK细胞呈圆形,细胞体积比较小,细胞散在分布,偶见细胞集落;培养第3天,PBMCs中NK细胞出现明显聚集,呈克隆样生长;培养第7天,PBMCs中NK细胞体积增大,有些细胞形态不规则;培养第14天,不规则形态NK细胞数明显增多。见图 1 (插页六)。
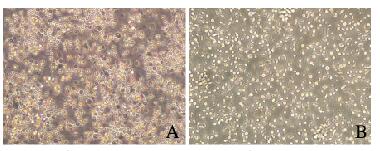
|
| A: Before expansion; B: 14 d after expansion. 图 1 扩增前后NK细胞形态表现 Fig. 1 Morphology of NK cells before and after expansion |
|
|
15例胃癌患者中1例代表性胃癌患者扩增前外周血中NK细胞百分比仅为14.1%(图 2A),经过14 d扩增后,NK细胞百分比达到89.6%(图 2B)。全部15例胃癌患者扩增前外周血中NK细胞百分比为(16.25±6.84)%;经过14 d扩增后,NK细胞百分比为(80.57±9.36)%(图 2C)。扩增后NK细胞百分比明显高于扩增前(P < 0.01)。
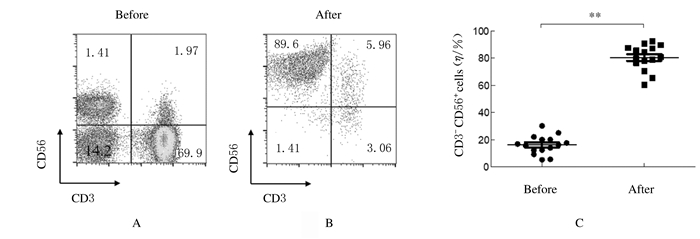
|
| A:Percentage of NK cells before expansion(from one patient); B: Percentage of NK cells after expansion(from one patient); C: Proportion graph of NK cells before and after expansion (from fifteen patients). 图 2 胃癌患者体外扩增前后NK细胞百分比 Fig. 2 Percentages of NK cells of patient with gastric cancer before and after amplification in vitro |
|
|
在体外扩增NK细胞的过程中,扩增前4 d,NK细胞增殖较慢;扩增第6天,NK细胞数明显增加;扩增第10天开始,NK细胞增殖速度呈线性增长,该阶段是NK细胞大量扩增期, 在体外扩增第14天,NK细胞数是扩增前的(596±152)倍。见图 3(每条线代表1例患者NK细胞体外扩增时NK细胞数的变化趋势)。
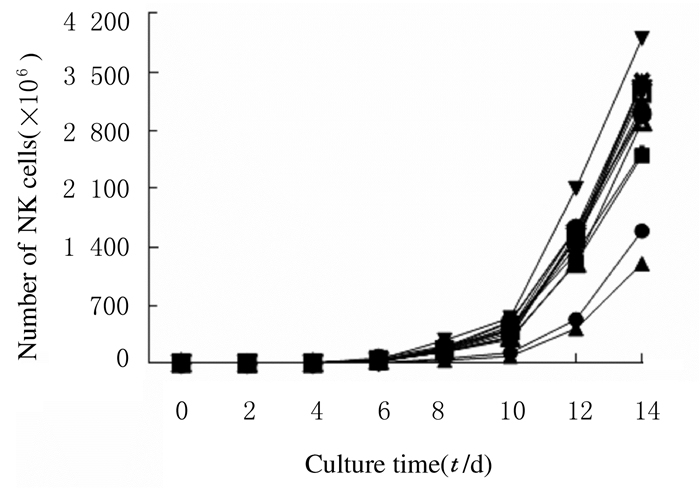
|
| 图 3 扩增过程中NK细胞增殖曲线 Fig. 3 Proliferation curres of NK cells during expansion |
|
|
分离胃癌患者PBMCs中扩增前后NK细胞,检测扩增前后NK细胞对胃癌细胞的杀伤活性。在效靶比为5:1时,扩增后NK细胞对胃癌细胞的杀伤活性(27.99%±6.39%)明显强于扩增前(12.32%±4.85%)(P < 0.01)。见图 4。
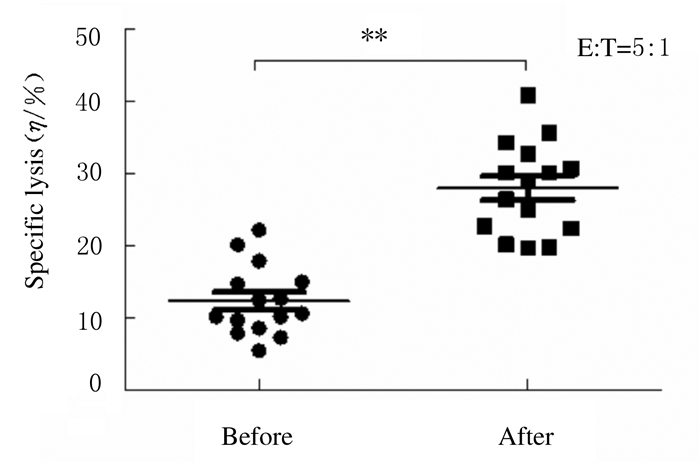
|
| 图 4 扩增前后NK细胞对胃癌细胞的杀伤活性 Fig. 4 Killing activities of NK cells against gastric carcinoma cells before and after expansion |
|
|
扩增后NK细胞表面活化性受体NKG2D(82.44%±15.57%)和DNAM-1(86.86%±11.91%)表达百分比明显高于扩增前(72.18%±13.98%和67.95%±15.01%,P < 0.01)。扩增后NK细胞表面抑制性受体KIR2DL1(18.65%±8.42%)和KIR3DL1(16.84%±7.92%)表达百分比明显低于扩增前(29.08%±12.41%和20.97%±9.48%,P < 0.01),表明扩增后NK细胞是活化程度更高的NK细胞。见图 5和图 6。
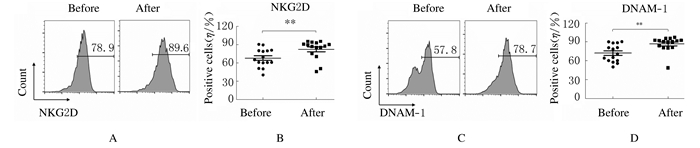
|
| A, C: Flow cytometry diagram; B, D: Scatter plot; A, B:Expression of NKG2D;C, D: Expression of DNAM-1. 图 5 扩增前后NK细胞表面活化性受体表达 Fig. 5 Expressions of activating receptors of NK cells before and after amplification |
|
|
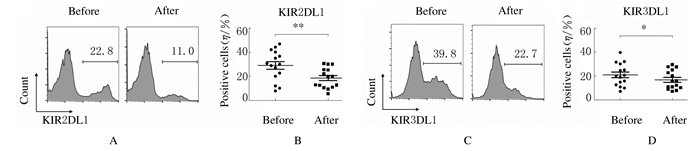
|
| A, C: Flow cytometry diagram; B, D: Scatter plot; A, B:Expression of KIR2DL1;C, D: Expression of KIR3DL1. 图 6 扩增前后NK细胞表面抑制性受体表达 Fig. 6 Expressions of inhibitory receptors of NK cells before and after amplification |
|
|
近年来,肿瘤的NK细胞疗法取得了较大进展[9-11]。NK细胞扩增方法主要有2种,一种是滋养层法,一种是细胞因子法[12]。滋养层法即采用致死剂量辐照的肿瘤细胞作为滋养层细胞刺激NK细胞增殖[13-15]。滋养层法虽然能够在短时间内获得大量、高纯度NK细胞,但是因为这种方法中加入了肿瘤细胞,存在肿瘤细胞继续增殖的风险,因此限制了该方法的临床应用。细胞因子法即采用细胞因子刺激NK细胞增殖[16-19],该方法去除了滋养层细胞成瘤的风险,且在一定的时间内也能获得大量、高纯度NK细胞,可以满足临床治疗需要。本研究将单克隆抗体和细胞因子联合来扩增NK细胞,经过14 d培养,NK细胞数能够满足临床治疗的需要,且扩增的NK细胞对胃癌细胞的杀伤作用明显强于胃癌患者外周血中的NK细胞,表明扩增的NK细胞可以用于胃癌的临床治疗。
NK细胞对肿瘤细胞杀伤可以通过3条途径:①分泌细胞毒性物质,包括穿孔素和颗粒酶;②分泌细胞因子,包括干扰素γ(interferon-γ,IFN-γ)和肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)等;③通过抗体依赖细胞介绍的细胞毒作用[20-23]。NK细胞抗肿瘤作用的发挥受到其表面活化分子和抑制分子的调控作用[24]。当活化信号强于抑制信号时,NK细胞被活化,进而发挥抗肿瘤作用[25-26]。本研究结果显示:扩增后NK细胞表面活化性受体NKG2D和DNAM-1表达明显高于扩增前,而抑制性受体KIR2DL1和KIR3DL1表达明显低于扩增前;本文作者发现:扩增后NK细胞对胃癌细胞的杀伤作用明显强于扩增前,这可能与扩增后NK细胞表面活化性受体增多而抑制性受体减少有关。当扩增的NK细胞与肿瘤细胞接触时,高表达的活性受体能够与其配体相互作用,并向NK细胞传递更多的活化信号,进而激活NK细胞,使NK细胞的抗肿瘤活性增强。
综上所述,经体外扩增后,NK细胞对胃癌细胞的杀伤活性明显增强,这可能与扩增的NK细胞表面活化性配体的表达升高、抑制性配体表达降低有关。本研究明确了扩增的NK细胞对胃癌细胞的杀伤作用及可能机制,为NK细胞治疗胃癌提供了理论基础。
| [1] |
FANG F, XIAO WH, TIAN Z G. NK cell-based immunotherapy for cancer[J]. Semin Immunol, 2017, 31: 37-54. DOI:10.1016/j.smim.2017.07.009 |
| [2] |
FEHNIGER T A, COOPER M A. Harnessing NK cell memory for cancer immunotherapy[J]. Trends Immunol, 2016, 37(12): 877-888. DOI:10.1016/j.it.2016.09.005 |
| [3] |
BASSANI B, BACI D, GALLAZZI M, et al. Natural killer cells as key players of tumor progression and angiogenesis:old and novel tools to divert their pro-tumor activities into potent anti-tumor effects[J]. Cancers (Basel), 2019, 11(4): E46. |
| [4] |
XIE S L, WU Z Y, NIU L Z, et al. Preparation of highly activated natural killer cells for advanced lung cancer therapy[J]. Oncol Targets Ther, 2019, 12: 5077-5086. DOI:10.2147/OTT.S201924 |
| [5] |
JUENGPANICH S, SHI L, IRANMANESH Y, et al. The role of natural killer cells in hepatocellular carcinoma development and treatment:A narrative review[J]. Transl Oncol, 2019, 12(8): 1092-1107. DOI:10.1016/j.tranon.2019.04.021 |
| [6] |
雷子颖, 王俞, 罗嘉莉, 等. 胃癌患者外周血T淋巴细胞亚群和NK细胞分析[J]. 消化肿瘤杂志(电子版), 2018, 10(4): 196-199. DOI:10.3969/j.issn.1674-7402.2018.04.006 |
| [7] |
HAN B, MAO F Y, ZHAO Y L, et al. Altered NKp30, NKp46, NKG2D, and DNAM-1 expression on circulating NK cells is associated with tumor progression in human gastric cancer[J]. J Immunol Res, 2018, 2018: 6248590. |
| [8] |
DU Y, WEI Y. Therapeutic potential of natural killer cells in gastric cancer[J]. Front Immunol, 2018, 9: 3095. |
| [9] |
REZVANI K, ROUCE R, LIU E L, et al. Engineering natural killer cells for cancer immunotherapy[J]. Mol Ther, 2017, 25(8): 1769-1781. DOI:10.1016/j.ymthe.2017.06.012 |
| [10] |
MARTIN-ANTONIO B, SUNE G, PEREZ-AMILL L, et al. Natural killer cells:angels and devils for immunotherapy[J]. Int J Mol Sci, 2017, 18(9): E1868. DOI:10.3390/ijms18091868 |
| [11] |
LI X M, HE C H, LIU C Z, et al. Expansion of NK cells from PBMCs using immobilized 4-1BBL and interleukin-21[J]. Int J Oncol, 2015, 47(1): 335-342. |
| [12] |
MUNTASELL A, OCHOA M C, CORDEIRO L, et al. Targeting NK-cell checkpoints for cancer immunotherapy[J]. Curr Opin Immunol, 2017, 45: 73-81. DOI:10.1016/j.coi.2017.01.003 |
| [13] |
PHAN M T, LEE S H, KIM S K, et al. Expansion of NK cells using genetically engineered k562 feeder cells[J]. Methods Mol Biol, 2016, 1441: 167-174. |
| [14] |
CHENG H Y, YE X, MA R Q, et al. Experiment research of natural killer cells amplification in vitro and the killing effect on ovarian cancer cells[J]. Zhonghua Fu Chan Ke Za Zhi, 2017, 52(8): 545-550. |
| [15] |
LAPTEVA N, DURETT A G, SUN J L, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications[J]. Cytotherapy, 2012, 14(9): 1131-1143. DOI:10.3109/14653249.2012.700767 |
| [16] |
LUHM J, BRAND J M, KORITKE P, et al. Large-scale generation of natural killer lymphocytes for clinical application[J]. J Hematother Stem Cell Res, 2002, 11(4): 651-657. DOI:10.1089/15258160260194794 |
| [17] |
CHILDS R W, BERG M. Bringing natural killer cells to the clinic:ex vivo manipulation[J]. Hematol Am Soc Hematol Edu Program, 2013, 2013: 234-246. DOI:10.1182/asheducation-2013.1.234 |
| [18] |
GRANZIN M, WAGNER J, KOHL U, et al. Shaping of natural killer cell antitumor activity by ex vivo cultivation[J]. Front Immunol, 2017, 8: 458. DOI:10.3389/fimmu.2017.00458 |
| [19] |
WAGNER J, PFANNENSTIEL V, WALDMANN A, et al. A two-phase expansion protocol combining interleukin (IL)-15 and IL-21 improves natural killer cell proliferation and cytotoxicity against rhabdomyosarcoma[J]. Front Immunol, 2017, 8: 676. DOI:10.3389/fimmu.2017.00676 |
| [20] |
VIVIER E, TOMASELLO E, BARATIN M, et al. Functions of natural killer cells[J]. Nat Immunol, 2008, 9(5): 503-510. DOI:10.1038/ni1582 |
| [21] |
KRASNOVA Y, PUTZ E M, SMYTH M J, et al. Bench to bedside:NK cells and control of metastasis[J]. Clin Immunol, 2017, 177: 50-59. DOI:10.1016/j.clim.2015.10.001 |
| [22] |
CAROTTA S. Targeting NK cells for anticancer immunotherapy:clinical and preclinical approaches[J]. Front Immunol, 2016, 7: 152. |
| [23] |
OCHOA M C, MINUTE L, RODRIGUEZ I, et al. Antibody-dependent cell cytotoxicity:immunotherapy strategies enhancing effector NK cells[J]. Immunol Cell Biol, 2017, 95(4): 347-355. DOI:10.1038/icb.2017.6 |
| [24] |
HILTON H G, PARHAM P. Missing or altered self:human NK cell receptors that recognize HLA-C[J]. Immunogenetics, 2017, 69(8/9): 567-579. |
| [25] |
TU M M, MAHMOUD A B, MAKRIGIANNIS A P. Licensed and unlicensed NK cells:differential roles in cancer and viral control[J]. Front Immunol, 2016, 7: 166. |
| [26] |
贺丽娜, 胡杨志, 周轩, 等. 沉默PROX1表达对胃癌AGS细胞增殖和侵袭能力的影响[J]. 郑州大学学报(医学版), 2019, 54(4): 559-563. |
 2020, Vol. 46
2020, Vol. 46


