扩展功能
文章信息
- 孟爽, 李迎杰, 臧晓珍, 赵乾芳, 张进, 李静
- MENG Shuang, LI Yingjie, ZANG Xiaozhen, ZHAO Qianfang, ZHANG Jin, LI Jing
- 敲减TLR2基因对结直肠癌细胞增殖的抑制作用及其机制
- Inhibitory effect of knockdown of TLR2 gene on proliferation of colorectal cancer cells and its mechanism
- 吉林大学学报(医学版), 2020, 46(02): 316-322
- Journal of Jilin University (Medicine Edition), 2020, 46(02): 316-322
- 10.13481/j.1671-587x.20200218
-
文章历史
- 收稿日期: 2019-10-17
结直肠癌是目前全球最常见的恶性肿瘤之一,是世界上第4位癌症死亡原因,到2030年,全球预计将有220万新发病例和110万死亡病例,严重威胁人类的健康,寻求分子靶点将成为结直肠癌治疗新的突破点[1-3]。Toll样受体(Toll-like receptors,TLRs)是一系列先天免疫传感器,不仅是形成先天免疫系统的重要组成部分,而且还可以激活适应性免疫系统, 广泛涉及传染性、炎症性和过敏性疾病以及致癌作用,其中Toll样受体2(Toll-like receptor-2, TLR2)分布表达于很多肿瘤细胞和组织中[4-8]。研究[6]表明:靶向敲减TLR2基因的表达能抑制多种肿瘤细胞的增殖。有研究[9]显示:结直肠癌患者肿瘤组织中TLR2高表达,TLR2在结直肠癌的发生发展中起着重要的作用。然而TLR2基因对结直肠癌细胞增殖作用的相关研究较少,且其作用机制仍然有待进一步阐明。抑制TLR2表达对抑制结直肠癌的发生发展也可能有重要的临床指导意义。慢病毒RNA干扰技术是进行靶基因表达抑制的核酸操作技术,目前已成为研究肿瘤基因治疗的重要工具。本研究采用慢病毒RNA干扰技术,敲减TLR2基因在结直肠癌HCT116细胞和HT29细胞中的表达,观察其对结直肠癌细胞增殖能力的影响,并对其相关分子机制进行探讨,以期为结直肠癌抗癌治疗提供新的靶点。
1 材料与方法 1.1 细胞、主要试剂和仪器人结直肠癌HCT116细胞和HT29细胞购自美国模式培养物集存库(American Type Culture Collection,ATCC)。McCoy’ s 5A培养液及胎牛血清(美国Gibco公司),慢病毒及转染试剂(上海吉凯基因化学技术公司),CCK-8试剂盒(日本同仁化学研究所),细胞周期试剂盒(上海贝博生物公司),单克隆抗体TLR2、磷脂酰肌醇3-激酶(PI3K)、磷酸化蛋白激酶B (p-Akt)、磷酸化核因子κB(p-NF-κB)、细胞周期蛋白D1(cyclin D1)和细胞周期蛋白D3(cyclin D3)(美国Cell Signaling Technology公司),电化学发光试剂(美国Azure Biosystems公司)。倒置光学显微镜(日本Olympus公司),NovoCyte流式细胞仪(杭州艾森生物有限公司)。
1.2 细胞培养和转染人结直肠癌HCT116细胞和HT29细胞在McCoy’ s 5A培养基中生长,补充10%胎牛血清及1%青链霉素。在含5%CO2、湿润无菌的37℃培养箱中培养。根据实验需求将对数生长的细胞经细胞计数后铺板过夜, 更换感染培养基, 细胞分为未感染对照组、阴性对照组和基因敲减组(TLR2-RNAi组)。根据感染复数(multiplicity of infection, MOI)为30加入最适量慢病毒及4%转染试剂进行感染, 未感染对照组不做任何处理,阴性对照组感染阴性对照iRNA, TLR2-RNAi组感染TLR2-RNAi;约12~16 h, 更换为用常规培养基继续培养。当细胞状态良好、细胞感染效率达到80%以上时, 加入1mg·L-1嘌呤霉素进一步筛选感染慢病毒的细胞,继续培养7~10 d后进行下游实验。转染效率=荧光阳性细胞数/筛选细胞总数×100%。
1.3 蛋白印迹法检测TLR2蛋白的表达收集各组细胞后,采用含PMSF的裂解液在冰上裂解30 min,在4℃、12 000 r·min-1离心30 min,然后采用BCA法测定上清液中蛋白质含量,制备总蛋白量相同的蛋白质样品。采用10% SDS-PAGE电泳后转移到PVDF膜上;在室温下采用5%脱脂牛奶封闭2 h,将一抗(TLR2和GAPDH)以1:1 000稀释后,于4℃孵育过夜;TBST洗膜3次,每次5 min后,用二抗1:5 000在室温下孵育2 h;TBST洗膜3次,每次5 min后通过ECL发光液使膜条带可视化,实验独立重复3次。以各实验组蛋白灰度值与相应内参灰度值的比值表示蛋白相对表达水平。
1.4 CCK-8法检测细胞增殖活性将上述各组HCT116细胞(每孔2 000个细胞)和HT29细胞(每孔5 000个细胞)接种于96孔培养板, 每组设置5个平行孔,置于37℃、5%CO2培养箱中培养;分别于细胞贴壁0、24、48和72 h后加入90μL培养基和10μLCCK-8溶液,同时设置只含有培养基和CCK-8溶液的空白对照孔,置于37℃、5%CO2培养箱孵育1 h, 于酶标仪450 nm波长处测定各组吸光度(A)值,实验独立重复3次,绘制生长曲线,计算细胞增殖活性。细胞增殖活性=实验组A值-空白对照组A值。
1.5 流式细胞术检测不同细胞周期细胞百分率将各组细胞以每孔5×105个接种于6孔培养板中,培养48 h后收集细胞;用预冷PBS缓冲液冲洗2次,300μL预冷PBS缓冲液重悬后,用700μL预冷的无水乙醇缓慢滴注并混匀,4℃固定过夜,1 000 r·min-1离心5 min后弃上清,预冷PBS缓冲液冲洗2次;500μL预冷PBS缓冲液重悬细胞后加入Rnase A溶液20 μL,37℃水浴30 min,1 000 r·min-1离心5 min后弃上清;加入500μL PI染色液重悬细胞,4℃避光染色30 min,采用流式细胞仪以标准程序检测,实验独立重复3次。NovoExpress 1.1.0软件进行细胞周期DNA含量分析,计算不同细胞周期细胞百分率。
1.6 蛋白印迹法检测细胞中信号通路及细胞周期相关蛋白表达水平将各组细胞的蛋白质样品采用10% SDS-PAGE电泳后转移到PVDF膜上;在室温下采用5%脱脂牛奶封闭2 h;根据蛋白相对分子质量进行切膜,在第一板胶转印后的PVDF膜上切出PI3K、cyclin D1和β-tubulin所在条带,另一板胶转印后的PVDF膜上切出p-Akt、cyclin D3和β-actin所在条带,第三板胶转印后的PVDF膜上切出p-NF-κB和β-actin所在条带,分别将相对应的一抗以1:1 000稀释后,于4℃孵育过夜;TBST洗膜3次,每次5 min,采用二抗1:5 000在室温下孵育2 h;TBST洗膜3次,每次5 min,通过ECL发光液使膜条带可视化,实验独立重复3次。以各实验组蛋白灰度值与相应内参灰度值的比值表示蛋白相对表达水平。
1.7 统计学分析采用SPSS 25.0统计软件进行统计学分析。各组细胞增殖活性,不同细胞周期细胞百分率,细胞中TLR2、PI3K、p-Akt、p-NF-κB、cyclin D1和cyclin D3蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析。以P<0.05为差异有统计学意义。
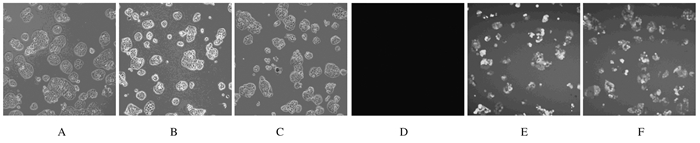
2 结果 2.1 慢病毒在结直肠癌细胞中的转染效率在构建慢病毒感染的稳转株过程中,荧光显微镜下观察结果显示:阴性对照组和TLR2-RNAi组HCT116细胞和HT29细胞的转染效率均达到80%以上,转染效率比较差异无统计学意义(P>0.05),可进行TLR2蛋白表达的验证。见图 1、图 2和表 1。

|
| A-C: Whitephotos; D-F: Fluorescence photos; A, D: Non-infection control group; B, E: Negative control group; C, F: TLR2-RNAi group. 图 1 荧光显微镜下观察慢病毒感染HCT116细胞5 d时的转染效率(×50) Fig. 1 Transfection efficiencies of HCT116 cells at 5 d after infection of lentivirus under fluorescence microscope (×50) |
|
|
|
| A-C: White photos; D-F: Fluorescence photos; A, D: Non-infection control group; B, E: Negative control group; C, F: TLR2-RNAi group. 图 2 荧光显微镜下观察慢病毒感染HT29细胞5 d时的转染效率(×50) Fig. 2 Transfection efficiencies of HT29 cells at 5 d after infection of lentivirus under fluorescence microscope (×50) |
|
|
| (n=3, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | Transfection efficiency of HCT116 cells | Transfection efficiency of HT29 cells | |||||||||||||||||||||||||||
| Non-infection control | 0.00±0.00 | 0.00±0.00 | |||||||||||||||||||||||||||
| Negative control | 86.33±4.54 | 88.33±2.25 | |||||||||||||||||||||||||||
| TLR2-RNAi | 86.50±2.78 | 87.17±2.52 | |||||||||||||||||||||||||||
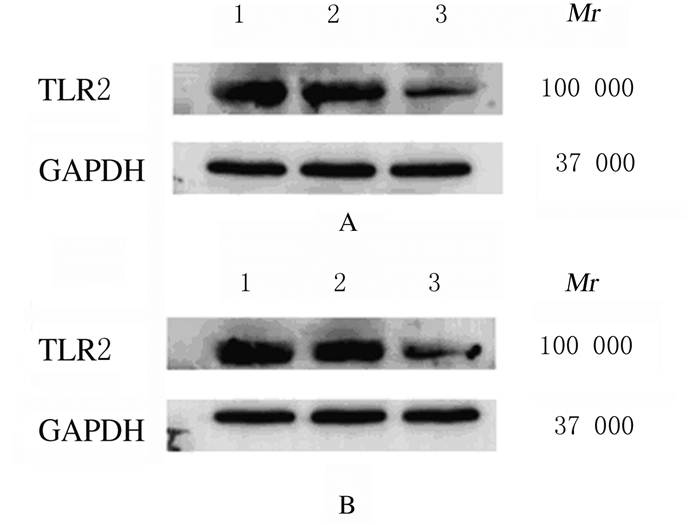
蛋白印迹法检测结果显示:未感染对照组、阴性对照组和TLR2-RNAi组HCT116细胞中TLR2蛋白表达水平分别为0.98±0.06、0.94±0.03和0.33±0.09;HT29细胞中TLR2蛋白表达水平分别为1.01±0.06、0.96±0.12和0.52±0.03。与未感染对照组和阴性对照组比较,TLR2-RNAi组HCT116细胞和HT29细胞中TLR2蛋白表达水平均明显降低(P < 0.01)。见图 3。
|
| Lane 1: Non-infection control group; Lane 2: Negative control group; Lane 3: TLR2-RNAi group. 图 3 蛋白印迹法检测各组HCT116(A)细胞和HT29(B)细胞中TLR2蛋白表达电泳图 Fig. 3 Electrophoregram of expressions of TLR2 protein in HCT116 cells(A)and HT29 cells(B)in various groups detected by Western blotting method |
|
|
CCK-8法检测结果显示:在感染24、48和72 h时,与未感染对照组和阴性对照组比较,TLR2-RNAi组HCT116细胞和HT29细胞增殖活性明显降低(P < 0.05)。见表 2。
| (n=3, x±s) | |||||||||||||||||||||||||||||
| Group | Proliferation activity of HCT116 cells | Proliferation activity of HT29 cells | |||||||||||||||||||||||||||
| (t/h) 0 | 24 | 48 | 72 | (t/h) 0 | 24 | 48 | 72 | ||||||||||||||||||||||
| Non-infection control | 0.191±0.009 | 0.547±0.015 | 0.785±0.025 | 1.153±0.032 | 0.320±0.010 | 0.486±0.015 | 0.675±0.019 | 0.883±0.016 | |||||||||||||||||||||
| Negative control | 0.194±0.010 | 0.532±0.009 | 0.800±0.031 | 1.134±0.051 | 0.308±0.009 | 0.478±0.021 | 0.670±0.021 | 0.924±0.022 | |||||||||||||||||||||
| TLR2-RNAi | 0.185±0.005 | 0.373±0.033*△ | 0.529±0.023*△ | 0.763±0.041*△ | 0.313±0.014 | 0.393±0.014*△ | 0.535±0.016*△ | 0.762±0.020*△ | |||||||||||||||||||||
| * P < 0.05 compared with non-infection control group; △ P < 0.05 compared with negative control group. A, D: Non-infection control group; B, E: Negative control group; C, F: TLR2-RNAi group. | |||||||||||||||||||||||||||||
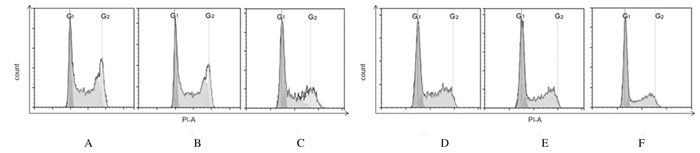
与未感染对照组和阴性对照组比较,TLR2-RNAi组HCT116细胞和HT29细胞中S期细胞百分率明显降低(P < 0.05),HCT116细胞和HT29细胞中G1期细胞百分率明显升高(P < 0.05)。见图 4和表 3。
|
| A, D: Non-infection control group; B, E: Negative control group; C, F: TLR2-RNAi group. 图 4 流式细胞术检测各组HCT116(A~C)和HT29(D~F)细胞周期 Fig. 4 Cell cycles of HCT116(A-C)and HT29(D-F)cells in various groups detected by flow cytometry |
|
|
| (n=3, x±s, η/%) | |||||||||||||||||||||||||||||
| Group | Percentage of HCT116 cells | Percentage of HT29 cells | |||||||||||||||||||||||||||
| G1 | S | G2 | G1 | S | G2 | ||||||||||||||||||||||||
| Non-infection control | 19.54±2.51 | 57.86±3.90 | 21.24±4.12 | 48.08±3.03 | 48.67±4.49 | 3.07±1.91 | |||||||||||||||||||||||
| Negative control | 18.40±3.51 | 57.27±6.37 | 22.65±9.68 | 46.21±4.74 | 49.64±3.16 | 3.58±1.94 | |||||||||||||||||||||||
| TLR2-RNAi | 47.52±5.18*△ | 33.92±3.60*△ | 17.33±7.44 | 58.28±2.31*△ | 38.40±1.35*△ | 3.28±2.11 | |||||||||||||||||||||||
| * P < 0.05 compared with non-infection control group; △ P < 0.05 compared with negative control group. | |||||||||||||||||||||||||||||
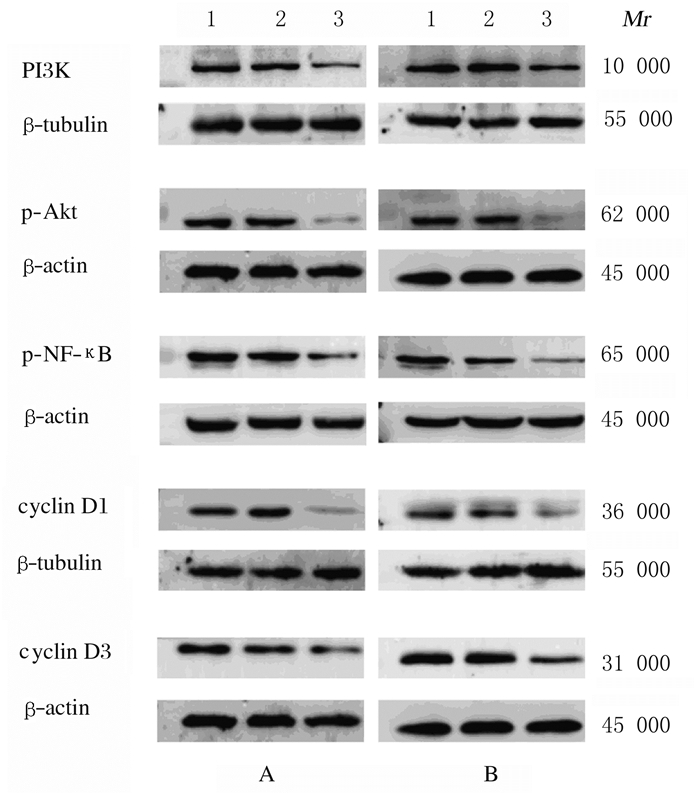
与未感染对照组和阴性对照组比较,TLR2-RNAi组HCT116细胞和HT29细胞中PI3K、p-Akt、p-NF-κB、cyclin D1和cyclin D3蛋白表达水平明显降低(P < 0.01)。见图 5和表 4。
|
| Lane 1: Non-infection control group; Lane 2: Negative control group; Lane 3: TLR2-RNAi group. 图 5 蛋白印迹法检测各组HCT116细胞(A)和HT29细胞(B)中细胞信号通路和细胞周期相关蛋白表达电泳图 Fig. 5 Electrophoregram of expressions of cell signaling pathway-related proteins and cell cycle-related proteins in HCT116 cells(A)and HT29 cells(B)in various groups detected by Western blotting method |
|
|
| (n=3, x±s) | |||||||||||||||||||||||||||||
| Group | Expression level of protein in HCT116 cells | Expression level of protein in HT29 cells | |||||||||||||||||||||||||||
| PI3K | p-Akt | p-NF-κB | Cyclin D1 | Cyclin D3 | PI3K | p-Akt | p-NF-κB | Cyclin D1 | Cyclin D3 | ||||||||||||||||||||
| Non-infection control | 1.05±0.11 | 1.23±0.05 | 1.06±0.10 | 0.89±0.02 | 1.19±0.12 | 1.16±0.07 | 1.19±0.13 | 1.07±0.14 | 1.03±0.09 | 1.19±0.05 | |||||||||||||||||||
| Negative control | 0.88±0.11 | 1.25±0.24 | 1.02±0.24 | 0.84±0.07 | 1.15±0.19 | 1.05±0.18 | 1.03±0.12 | 0.90±0.12 | 0.87±0.10 | 1.06±0.09 | |||||||||||||||||||
| TLR2-RNAi | 0.41±0.03*△ | 0.49±0.17*△ | 0.29±0.15*△ | 0.35±0.08*△ | 0.57±0.10*△ | 0.59±0.08*△ | 0.50±0.14*△ | 0.55±0.11*△ | 0.44±0.10*△ | 0.53±0.13*△ | |||||||||||||||||||
| * P < 0.01 compared with non-infection control group; △ P < 0.01 compared with negative control group. | |||||||||||||||||||||||||||||
研究[10]显示:在大多数胃癌患者中TLR2蛋白呈高水平表达,且TLR2蛋白高水平表达与增殖基因相关并提示预后不良,也有研究[11]提示TLR2促进胃癌的发生;TLR2在胰腺癌、套细胞淋巴瘤和乳腺癌中也有促进肿瘤细胞增殖的作用[12-14];研究[5-6, 9, 15-19]表明:结直肠癌组织通常比来自同一患者的正常结肠黏膜具有更高的TLR2基因表达水平,且在结直肠癌中TLR2基因和TLR4基因的激活也表现为促进肿瘤细胞的增殖。本研究采用慢病毒转染敲减结直肠癌细胞中的TLR2基因,在第24、48和72小时与未感染对照组比较,阴性对照组HCT116细胞和HT29细胞增殖活性差异无统计学意义,TLR2-RNAi组细胞增殖活性明显降低,且具有统计学意义。上述结果提示:敲减TLR2基因表达能够明显抑制结直肠癌细胞增殖。本研究中细胞周期检测结果显示:与未感染对照组和阴性对照组比较,TLR2-RNAi组HCT116细胞和HT29细胞中G1期细胞百分率明显升高,且具有统计学意义。提示敲减TLR2基因表达能够抑制结直肠癌细胞增殖,导致细胞阻滞在G1期,从而明显抑制结直肠癌细胞周期。上述结果进一步说明敲减TLR2基因可能抑制结直肠癌细胞的增殖作用。
包括结直肠癌在内的各种肿瘤发病机制均是多元化的,并且通过多条细胞信号通路的协同调控。PI3K/Akt信号通路参与增殖、分化、凋亡和葡萄糖转运等多种细胞功能的调节,NF-κB信号通路是重要的炎症信号通路[5]。cyclin D1和cyclin D3是位于PI3K/Akt和NF-κB信号通路的下游蛋白, NF-κB属于Rel家族的转录因子,其参与免疫和炎症反应,NF-κB的p65亚基是cyclin D1基因的有效转录激活因子,是cyclin D1启动子转录激活所必需[14, 19]。在细胞增殖进程中cyclins起正性调节作用。cyclin D1与cyclin D3的主要功能是促进细胞增殖,通过结合并激活G1期特有的周期蛋白依赖性激酶CDK4,G1期周期抑制蛋白(Rb)被磷酸化,磷酸化的Rb蛋白从其所结合的E2F转录因子上解离,从而释放出E2F转录因子,E2F与相应的含有E2F调节元件的DNA结合,促进其转录,从而推动细胞周期由G1期进入到S期[20-21]。本研究结果显示:与未感染对照组和阴性对照组比较,TLR2-RNAi组HCT116和HT29细胞中PI3K、p-Akt、p-NF-κB、cyclin D1和cyclin D3蛋白表达水平明显降低,差异有统计学意义。这些结果表明敲减TLR2基因可能通过调控PI3K/Akt和NF-κB细胞信号通路及细胞周期相关蛋白cyclin D1和cyclin D3的表达而抑制结直肠癌细胞的增殖。
综上所述,敲减TLR2基因可以通过调控PI3K/Akt和NF-κB细胞信号通路及细胞周期相关蛋白cyclin D1和cyclin D3的表达而抑制结直肠癌细胞的增殖。本研究证明了TLR2在结直肠癌中可能起着关键性的作用,并且可能为结直肠癌的抗癌治疗提供新的靶点。
| [1] |
ARNOLD M, SIERRA M S, LAVERSANNE M, et al. Global patterns and trends in colorectal cancer incidence and mortality[J]. Gut, 2017, 66: 683-691. DOI:10.1136/gutjnl-2015-310912 |
| [2] |
SCHREUDERS E H, RUCO A, RABENECK L, et al. Colorectal cancer screening:a global overview of existing programmes[J]. Gut, 2015, 64(10): 1637-1649. DOI:10.1136/gutjnl-2014-309086 |
| [3] |
LINNEKAMP J F, WANG X, MEDEMA J P, et al. Colorectal cancer heterogeneity and targeted therapy:a case for molecular disease subtypes[J]. Cancer Res, 2015, 75(2): 245-249. |
| [4] |
LIU Y, YIN H, ZHAO M, et al. TLR2 and TLR4 in autoimmune diseases:a comprehensive review[J]. Clin Rev Allergy Immunol, 2014, 47(2): 136-147. DOI:10.1007/s12016-013-8402-y |
| [5] |
LIU Y D, JI C B, LI S B, et al. Toll-like receptor 2 stimulation promotes colorectal cancer cell growth via PI3K/Akt and NF-κB signaling pathways[J]. Int Immunopharmacol, 2018, 9: 375-383. |
| [6] |
LU C C, KUO H C, WANG F S, et al. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer[J]. Int J Mol Sci, 2015, 16(1): 159-177. |
| [7] |
MEDVEDEV A E. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer[J]. J Interferon Cytokine Res, 2013, 33(9): 467-484. DOI:10.1089/jir.2012.0140 |
| [8] |
HERNANDEZ C, HUEBENER P, SCHWABE R F. Damage-associated molecular patterns in cancer:a double-edged sword[J]. Oncogene, 2016, 35(46): 5931-5941. DOI:10.1038/onc.2016.104 |
| [9] |
PROENCA M A, DE OLIVEIRA J G, CADAMURO A C, et al. TLR2 and TLR4 polymorphisms influence mRNA and protein expression in colorectal cancer[J]. World J Gastroenterol, 2015, 21(25): 7730-7741. DOI:10.3748/wjg.v21.i25.7730 |
| [10] |
KENNEDY C L, NAJDOVSKA M, TYE H, et al. Differential role of MyD88 and Mal/TIRAP in TLR2-mediated gastric tumourigenesis[J]. Oncogenes, 2014, 33(19): 2540-2546. DOI:10.1038/onc.2013.205 |
| [11] |
WEST A C, TANG K, TYE H, et al. Identification of a TLR2-regulated gene signature associated with tumor cell growth in gastric cancer[J]. Oncogenes, 2017, 36(36): 5134-5144. DOI:10.1038/onc.2017.121 |
| [12] |
GRIMMIG T, MOENCH R, KRECKEL J, et al. Toll like receptor 2, 4, and 9 signaling promotes autoregulative tumor cell growth and VEGF/PDGF expression in human pancreatic cancer[J]. Int J Mol Sci, 2016, 17(12): e2060. DOI:10.3390/ijms17122060 |
| [13] |
SCHEEREN F A, KUO A H, et al. A cell-intrinsic role for TLR2-MYD88 in intestinal and breast epithelia and oncogenesis[J]. Nat Cell Biol, 2014, 16(12): 1238-1248. DOI:10.1038/ncb3058 |
| [14] |
MASTORCI K, MURARARO E, PASINI E, et al. Toll-like receptor 1/2 and 5 ligands enhance the expression of cyclin D1 and D3 and induce proliferation in mantle cell lymphoma[J]. PLoS One, 2016, 11(4): e0153823. DOI:10.1371/journal.pone.0153823 |
| [15] |
LI X T, QIAN D M, JU F, et al. Upregulation of Toll-like receptor 2 expression in colorectal cancer infected by human cytomegalovirus[J]. Oncol Lett, 2015, 9(1): 365-370. DOI:10.3892/ol.2014.2621 |
| [16] |
GUO H, CHEN Y, HU X, et al. The regulation of Toll-like receptor 2 by miR-143 suppresses the invasion and migration of a subset of human colorectal carcinoma cells[J]. Mol Cancer, 2013, 12: 77. DOI:10.1186/1476-4598-12-77 |
| [17] |
WANG M J, PING J, LI Y, et al. Prognostic significance and molecular features of colorectal mucinous adenocarcinomas:a strobe-compliant study[J]. Medicine (Baltimore), 2015, 94(51): e2350. DOI:10.1097/MD.0000000000002350 |
| [18] |
SEMLALI A, REDDY P N, ARAFAH M, et al. Expression and polymorphism of Toll-like receptor 4 and effect on NF-κB mediated inflammation in colon cancer patients[J]. PLoS One, 2016, 11(1): e0146333. DOI:10.1371/journal.pone.0146333 |
| [19] |
GUTTRIDGE D C, ALBANESE C, REUTHER J Y, et al. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1[J]. Mol Cell Biol, 1999, 19(8): 5785-5799. DOI:10.1128/MCB.19.8.5785 |
| [20] |
WILLIAMS G H, STOECER K. The cell cycle and cancer[J]. J Pathol, 2012, 226(2): 352-364. DOI:10.1002/path.3022 |
| [21] |
LIU Y Y, FAN C H, PU L, et al. Phloretin induces cell cycle arrest and apoptosis of human glioblastoma cells through the generation of reactive oxygen species[J]. J Neurooncol, 2016, 128(2): 217-223. DOI:10.1007/s11060-016-2107-z |
 2020, Vol. 46
2020, Vol. 46


