扩展功能
文章信息
- 和莹莹, 薛金慧, 赵娜
- HE Yingying, XUE Jinhui, ZHAO Na
- 大黄酸对非小细胞肺癌A549细胞增殖、迁移和侵袭能力的影响及其机制
- Effects of rhein on proliferation, migration and invasion abilities of non-small cell lung cancer A549 cells and their mechanisms
- 吉林大学学报(医学版), 2020, 46(02): 302-308
- Journal of Jilin University (Medicine Edition), 2020, 46(02): 302-308
- 10.13481/j.1671-587x.20200216
-
文章历史
- 收稿日期: 2019-05-22
肺癌是世界上死亡率最高的恶性肿瘤,其中非小细胞肺癌(non-small cell lung cancer, NSCLC)约占肺癌总数的80%[1-3]。NSCLC具有难发现、易转移和高复发的特点,虽然手术联合放化疗和靶向治疗在NSCLC中取得了一定效果,但晚期患者生存率偏低[4-7],同时现有治疗手段和药物均伴有严重的不良反应,明显降低患者的生存质量[8],采用高效且低毒的药物治疗是近年来NSCLC临床研究的焦点。大黄酸是中药大黄的主要活性成分之一,具有抗氧化、抗炎和抗菌等多种药理活性[9-12]。研究[13-16]表明:大黄酸具有抗癌作用,其作用机制主要包括促进细胞凋亡、抑制细胞增殖和抑制细胞迁移及侵袭。目前国内外关于大黄酸对抗NSCLC增殖和抗转移作用的研究未见报道。本研究选择NSCLC A549细胞作为研究对象,通过检测大黄酸对A549细胞增殖、迁移和侵袭能力的影响及相关凋亡蛋白的表达水平,分析其作用机制,为开发抗肿瘤天然药物提供基础依据。
1 材料与方法 1.1 细胞、主要试剂和仪器人NSCLC A549细胞株购自中山大学附属肿瘤医院细胞培养物中心。大黄酸和四甲基偶氮唑蓝(MTT)均购自美国Sigma公司,Bradford蛋白质浓度定量试剂盒和DMEM培养基胎牛血清(fetal bovine serum, FBS)购自菲博生物科技有限公司,兔抗人半胱氨酸蛋白酶3(cysteine protease-3, Caspase-3)、半胱氨酸蛋白酶9(cysteine protease-9, Caspase-9)、细胞色素C(cytochrome C, Cyt-C)和凋亡诱导因子(apoptosis induced factor, AIF)抗体购自美国Bio-Rad公司。凝胶成像仪和电泳仪(美国Bio-Rad公司)。
1.2 细胞培养细胞生长培养条件:置于含10%FBS的DMEM培养基,37℃、5% CO2培养箱中进行孵育,0.5%胰酶消化液消化传代,每3d 1次,实验均采用对数生长期细胞。
1.3 MTT法检测A549细胞增殖能力取对数生长期的A549细胞以每孔4×103个接种于96孔板中,于37℃、5% CO2条件下孵育24 h,贴壁后,弃去培养液,实验设对照组和低、中及高剂量大黄酸组,每个浓度设6孔。低、中和高剂量大黄酸组分别加入5、10和20 μmol· L-1大黄酸;对照组加入0.1% DMSO培养液。分别培养24、48和72 h后,弃去培养液,于各孔加入20 μL MTT溶液(5 mg· L-1)继续培养4 h,弃去上清液,加入150 μL DMSO,置于摇床室温避光振荡10 min,采用酶标仪检测530nm处各孔吸光度(A)值,以细胞增殖率表示细胞增殖能力。细胞增殖率=实验孔A值/对照孔A值×100%。
1.4 流式细胞术FITC-Annexin Ⅴ/PI双染色法检测细胞凋亡率采用低、中和高剂量(5、10和20 μmol· L-1)大黄酸处理A549细胞24 h后,按FITC-Annexin Ⅴ/PI凋亡检测试剂盒说明书操作,加入100 μL细胞悬液、5 μL Annexin Ⅴ-FITC和10 μL PI混匀,室温避光孵育30 min。加入5 μL PI(10 mg· L-1),在黑暗中再次孵育10 min。上流式细胞仪采用Cell Quest软件测定各组细胞凋亡率。
1.5 流式细胞术分析细胞周期取对数生长期A549细胞,稀释至1×106 L-1,接种于6孔板,每孔1 mL,随机分为对照组和低、中及高剂量大黄酸组(5、10和20 μmol· L-1),每组3次平行试验。细胞经大黄酸处理24 h后,对照组和大黄酸组均经胰蛋白酶消化,PBS缓冲液冲洗,重悬在2 mL 70%乙醇中,4℃固定过夜。PBS缓冲液固定细胞,然后加入500 μL含37 mmol· L-1EDTA和0.1% Triton X-100的PBS缓冲液,最后加入100 μL PI (400 mg· L-1),4℃避光保存,采用流式细胞仪分析不同细胞周期A549细胞百分比。
1.6 Western blotting法检测各组A549细胞中Caspase-3、Caspase-9、Cyt-C和AIF蛋白表达水平低、中和高剂量大黄酸组A549细胞分别采用5、10和20 μmol· L-1大黄酸处理48 h,收集A549细胞,采用RIPA细胞裂解液裂解30 min,超声破碎、离心分离后取上清液,Bradford法进行总蛋白质定量,上样量为40 μg,120 V电泳分离蛋白,PVDF膜上转膜2 h,5%脱脂奶粉封闭1h,加入一抗(1:1000),4℃孵育过夜,加入二抗(1:1000稀释)37℃孵育2 h,ECL法显影,采用Image J软件分析条带积分光密度,以β-actin作为内参,行半定量分析,计算蛋白表达水平。蛋白表达水平=蛋白积分光密度/β-actin积分光密度。
1.7 单层细胞划痕实验检测A549细胞迁移距离在6孔板中加入对数生长期的A549细胞(每孔约3×105个细胞),37℃孵育过夜后用移液枪头垂直划痕。PBS缓冲液冲洗细胞去除划下的细胞,低、中和高剂量大黄酸组分别加入5、10和20 μmol· L-1大黄酸的无血清培养基,对照组加入不含药物的无血清培养基,在37℃、5% CO2培养箱培养,于0和24 h时拍照,每孔3个平行拍照,观察0和24 h划痕宽度,计算细胞迁移距离。细胞迁移距离(μm)=0 h划痕宽度-24 h划痕宽度。以细胞迁移距离表示细胞迁移能力。
1.8 Transwell实验检测A549细胞侵袭数量取对数生长期A549细胞,以血清培养基洗涤,调整细胞密度至1×105 mL-1,取细胞悬液200 μL加入Transwell上室,低、中和高剂量大黄酸组分别加入5、10和20 μmol· L-1大黄酸,对照组加入不含药物的细胞混悬液,每组3复孔。Transwell下室加入含FBS的完全培养基。培养24 h,上室内肿瘤细胞向下室聚集。24 h后,取出培养板,用棉签擦去上室内的细胞,下室细胞以甲醇固定,0.1%结晶紫染色,冲洗封片后,显微镜下随机取5个视野观察侵袭细胞数量。
1.9 统计学分析采用SPSS 17.0统计软件进行统计学分析。各组细胞增殖率、细胞凋亡率、不同细胞周期A549细胞百分比和Caspase-3、Caspase-9、Cyt-C及AIF蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组A549细胞增殖率与对照组比较,低、中和高剂量大黄酸组处理24、48及72 h后A549细胞增殖率均明显降低(P < 0.05);与低剂量大黄酸组比较,中和高剂量大黄酸组A549细胞增殖率均明显降低(P < 0.05);与中剂量大黄酸组比较,高剂量大黄酸组细胞增殖率明显降低(P < 0.05)。见表 1。
| Group | Proliferation rate of A549 cells | ||
| (t/h) 24 | 48 | 72 | |
| Control | 0.96±0.14 | 1.08±0.15 | 1.33±0.23 |
| Rhein | |||
| Low dose | 0.78±0.12*# | 0.96±0.11*# | 1.06±0.21*# |
| Medium dose | 0.67±0.07*△ | 0.76±0.04*△ | 0.85±0.08*△ |
| High dose | 0.56±0.05*△# | 0.56±0.07*△# | 0.62±0.02*△# |
| * P < 0.05 compared with control group; △ P < 0.05 compared with low dose of rhein group; # P < 0.05 compared with medium dose of rhein group. | |||
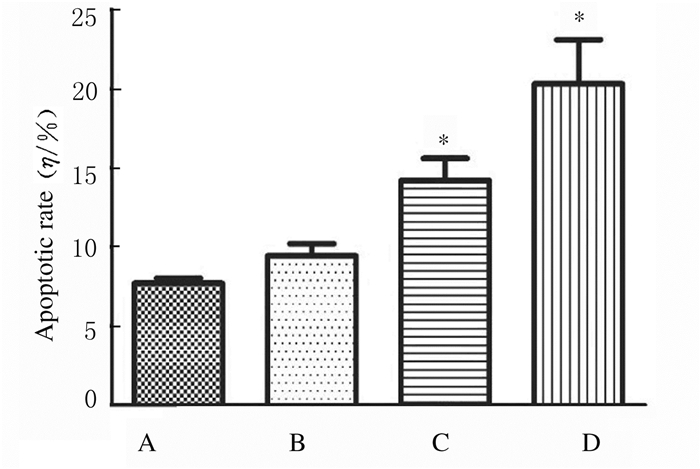
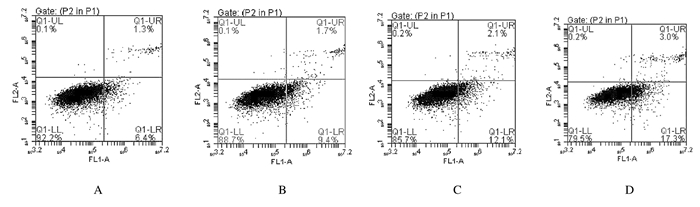
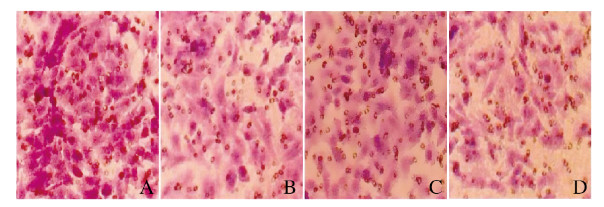
处理24 h后,与对照组比较,低剂量大黄酸组细胞凋亡率升高,但差异无统计学意义(P>0.05),中和高剂量大黄酸组细胞凋亡率均明显升高(P < 0.05)。见图 1和图 2。
|
| A:Control group; B-D:Low, medium and high doses of rhein groups. 图 1 各组A549细胞凋亡率 Fig. 1 Apoptotic rates of A549 cells in various groups |
|
|
|
| A:Control group; B-D:Low, medium and high doses of rhein groups.. 图 2 流式细胞术检测各组A549细胞凋亡率 Fig. 2 Apoptotic rates of A549 cells in various groups defected by flow eytometry |
|
|
处理24 h后,与对照组比较,高剂量大黄酸组G1期A549细胞百分比明显减少(P < 0.05),G2期A549细胞百分比明显升高(P < 0.05)。见图 3和图 4。

|
| A:Control group; B-D:Low, medium and high doses of rhein groups.. 图 3 流式细胞术检测各组A549细胞周期 Fig. 3 Cell cycles of A549 cells in various groups detected by flow cytometry |
|
|

|
| A:Control group; B-D:Low, medium and high doses of rhein groups. 图 4 各组不同细胞周期A549细胞百分比 Fig. 4 Percentages of A549 cells at different cell cycles in various groups |
|
|
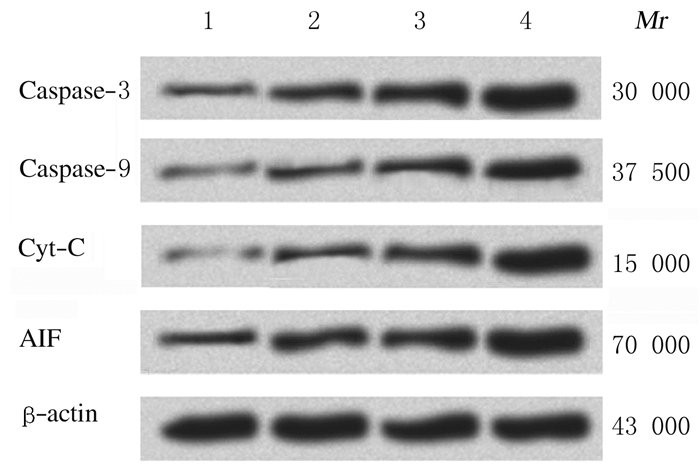
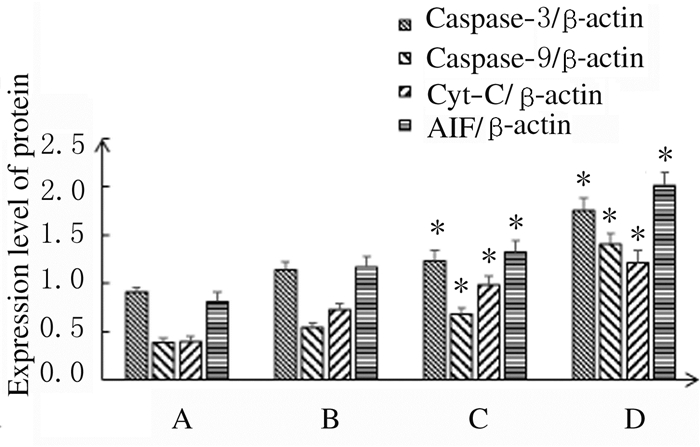
处理48 h后,与对照组比较,中和高剂量大黄酸组A549细胞中Caspase-3、Caspase-9、Cyt-C和AIF蛋白表达水平明显升高(P < 0.05)。见图 5和图 6。
|
| Line 1: Control group; Line 2-4: Low, medium and high doses of rhein groups. 图 5 Western blotting法检测各组A549细胞中Caspase-3, Caspase-9, Cyt-C和AIF蛋白表达电泳图 Fig. 5 Electrophoregram of expressions of Caspase-3, Caspase-9, Cyt-C and AIF proteins in A549 cells in various groups detected by Western blotting method |
|
|
|
| A: Control group; B-D: Low, medium and high doses of rhein groups.*P < 0.05 compared with control group. 图 6 各组A549细胞中Caspase-3、Caspase-9、Cyt-C和AIF蛋白表达水平 Fig. 6 Expression levels of Caspase-3, Caspase-9, Cyt-C and AIF proteins in A549 cells in various groups |
|
|
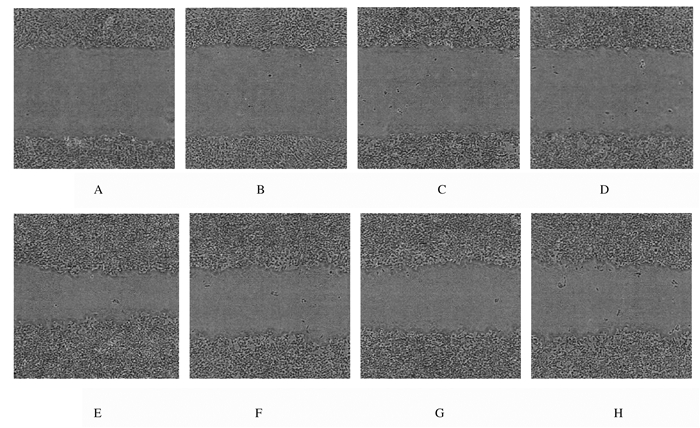
处理24 h后,与对照组比较,低、中和高剂量大黄酸组细胞迁移距离明显减少,侵袭细胞数量明显降低。见图 7和图 8(插页四)。
|
| A, E: Control group; B, F: Low dose of rhein group; C, G:Medium dose of rhein group; D, H: High dose of rhein group; A-D:0 h; E-H:24 h. 图 7 划痕实验检测各组A549细胞迁移能力 Fig. 7 Migration abilities of A549 cells in various groups detected by scratch test |
|
|
|
| A: Control group; B-D: Low, medium and high doses of rhein groups. 图 8 Transwell法检测A549细胞侵袭能力(结晶紫, × 200) Fig. 8 Invasion abilities of A549 cells in various groups detected by Transwell assay(Crystal violet, × 200) |
|
|
肺癌是最常见的恶性肿瘤之一,其中NSCLC是最常见的肺癌类型[17]。促进癌细胞凋亡是肺癌治疗的常用手段之一,细胞凋亡主要分为3种途径:死亡受体途径、线粒体途径和内质网途径。线粒体介导的凋亡相关因子主要包括Caspase、Cyt-C和AIF等[18]。Caspase家族通过破坏DNA、诱导细胞形成凋亡小体等方式,在细胞凋亡中发挥重要作用,主要包括Caspase-1、Caspase-3和Caspase-9。Caspase-3参与细胞凋亡起始,在细胞凋亡中发挥关键的作用,主要通过水解PARP,使受PARP负调控的因子升高,破坏核小体间DNA,从而引起细胞凋亡[19]。Cyt-C是线粒体受凋亡刺激后释放到细胞质的可溶性蛋白,是线粒体途径诱导细胞凋亡的关键因素,通过Caspase依赖途径来诱导细胞凋亡[20-21]。Cyt-C在胞浆内与凋亡酶激活因子1 (apoptotic protease activating factor-1,Apaf-1)结合,形成复合物,从而暴露出Caspase-9的结合位点,再聚合成三者的复合物,即“凋亡体”。而活化后的Caspase-9引起Caspase级联反应,再激活下游的Caspase-3,Caspase-3继而引起核小体间DNA裂解,最终导致细胞凋亡[22]。AIF属于细胞线粒体细胞膜间隙的黄素蛋白,为诱发细胞凋亡因子。线粒体在受到凋亡刺激后,膜通透性增加,AIF被释放到胞浆,再定向转移至细胞核,促使DNA裂解[23-25]。研究[26]发现:Caspase通路与AIF既互相独立,又多水平交叉,Caspase通路可诱发AIF释放,同时AIF的释放又可促进Cyt-C的释放。
近年来,天然产物的抗肿瘤活性为研究者所关注,其中凋亡诱导和增殖活性抑制是其治疗癌症的研究热点[27-28]。这些天然产物通过促进NSCLC细胞凋亡,有效地抑制恶性肿瘤的生长且不良反应少。大黄酸是中药大黄中的主要活性成分之一,已被证实对各种恶性肿瘤细胞具有较好的诱导凋亡和抗增殖的作用[29-30],但目前有关大黄酸对肺癌细胞的影响及机制的研究较少。本研究结果显示:与对照组比较,低、中和高剂量大黄酸组处理24、48和72 h后A549细胞增殖率明显下降,呈时间和剂量依赖性。中和高剂量大黄酸组A549细胞凋亡率明显升高,说明不同剂量大黄酸均能抑制A549细胞增殖,诱导细胞凋亡,使细胞周期阻滞在G2期,并具有明显的时间和剂量依赖性。本研究中Western blotting法检测结果表明:A549细胞经大黄酸处理48 h后,与对照组比较,低、中和高剂量大黄酸组A549细胞中Caspase-3、Caspase-9、Cyt-C及AIF蛋白表达水平均明显升高,说明大黄酸的抗肿瘤作用可能是通过调控蛋白表达途径诱导细胞凋亡。划痕实验和Transwell实验检测结果表明:大黄酸处理24h后,A549细胞的迁移和侵袭能力均受到明显抑制。
综上所述,大黄酸可通过调控细胞凋亡相关蛋白的表达有效抑制NSCLC细胞的增殖、迁移和侵袭,本研究结果为临床新药开发提供了理论依据。
| [1] |
ROACH M C, ROBINSON C G, DEWEES T A, et al. Stereotactic body radiation therapy for central early-stage NSCLC:results of a prospective phase Ⅰ/Ⅱ trial[J]. J Thorac Oncol, 2018, 13(11): 1727-1732. |
| [2] |
ISLAMI F, FERLAY J, LORTET-TIEULENT J, et al. International trends in anal cancer incidence rates[J]. Int J Epidemiol, 2017, 46(3): 924-938. |
| [3] |
POSTMUS P E, KERR K M, OUDKERK M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC):ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Anna Oncol, 2017, 28(4): 1-21. |
| [4] |
SARVI S, MACKINNON A C, AVLONITIS N, et al. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist[J]. Cancer Res, 2014, 74(5): 1554-1565. |
| [5] |
SANTANA-DAVILA R, CHOW L Q. The use of combination immunotherapies as front-line therapy for non-small-cell lung cancer[J]. Future Oncol, 2018, 14(3): 191-194. |
| [6] |
WATANABE M, KAWAGUCHI T, ISA S, et al. Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-small cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR[J]. Clin Cancer Res, 2015, 21(15): 3552-3560. |
| [7] |
CHRISTENSEN N L, JEKUNEN A, HEINONEN S, et al. Lung cancer guidelines in Sweden, Denmark, Norway and Finland:a comparison[J]. Acta Oncol, 2017, 56(7): 943-948. |
| [8] |
SORIA J, WU Y L, NAKAGAWA K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS):a phase 3 randomised trial[J]. Lancet Oncol, 2015, 16(8): 990-998. |
| [9] |
GUO M Z, LI X S, XU H R, et al. Rhein inhibits liver fibrosis induced by carbon tetrachloride in rats[J]. Acta Pharmacol Sin, 2002, 23(8): 739-744. |
| [10] |
NAMVARAN A, FAZELI M, FARAJNIA S, et al. Apoptosis and caspase 3 pathway role on anti-proliferative effects of scrophulariaoxy sepala methanolic extract on Caco-2 cells[J]. Drug Res(Stuttg), 2017, 67(9): 547-552. |
| [11] |
GAO Y, CHEN X, FANG L, et al. Rhein exerts pro- and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages[J]. Free Radic Biol Med, 2014, 72: 104-112. |
| [12] |
HU G Y, PENG C, XIE X F, et al. Availability, pharmaceutics, security, pharmacokinetics, and pharmacological activities of patchouli alcohol[J]. Evid Based Complement Alternat Med, 2017, 2017: 4850612. |
| [13] |
ZHOU G G, PENG F H, ZHONG Y P, et al. Rhein suppresses matrix metalloproteinase production by regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian carcinoma cells[J]. Int J Oncol, 2017, 50(3): 933-941. |
| [14] |
LIU S, WANG J, SHAO T, et al. The natural agent rhein induces β-catenin degradation and tumour growth arrest[J]. J Cell Mol Med, 2018, 22(1): 589-599. |
| [15] |
钱文斌. 大黄酸对乳腺癌肿瘤细胞HER-2蛋白表达的影响研究[J]. 中华中医药学刊, 2015, 33(1): 224-226. |
| [16] |
CHO J H, CHAE J I, SHIM J H. Rhein exhibits antitumorigenic effects by interfering with the interaction between prolyl isomerase Pin1 and c-Jun[J]. Oncol Rep, 2017, 37(3): 1865-1872. |
| [17] |
SHI Y K, AU J S, THONGPRASERT S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology(PIONEER)[J]. J Thorac Oncol, 2014, 9(2): 154-162. |
| [18] |
WANG L L, MA G Y, ZHANG Y B, et al. Effect of mitochondrial cytochrome c release and its redox state on the mitochondrial-dependent apoptotic cascade reaction and tenderization of yak meat during postmortem aging[J]. Food Res Int, 2018, 111: 488-497. |
| [19] |
VANDE VELDE C, CIZEAU J, DUBIK D, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore[J]. Mol Cell Biol, 2000, 20(15): 5454-5468. |
| [20] |
LIU X, KIM C N, YANG J, et al. Induction of apoptotic program in cell free extracts:requirement for dATP and cytochrome C[J]. Cell, 1996, 86(1): 147-157. |
| [21] |
SINGH M H, BROOKE S M, ZEMLYAK I, et al. Evidence for caspase effects on release of cytochrome c and AIF in a model of ischemia in cortical neurons[J]. Neurosci Lett, 2010, 469(2): 179-183. |
| [22] |
YU X C, WANG L, ACEHAN D, et al. Three-dimensional structure of a double apoptosome formed by the Drosophila Apaf-1 related killer[J]. J Mol Biol, 2006, 355(3): 577-589. |
| [23] |
MISEVICIENE L, ANUSEVICIUS Z, SARLAUSKAS J, et al. Redox reactions of the FAD-containing apoptosis-inducing factor (AIF) with quinoidal xenobiotics:a mechanistic study[J]. Arch Biochem Biophys, 2011, 512(2): 183-189. |
| [24] |
SCOVASSI A I, SOLDANI C, VENERONI P, et al. Changes of mitochondria and relocation of the apoptosis-inducing factor during apoptosis[J]. Ann N Y Acad Sci, 2009, 117(1): 12-17. |
| [25] |
BENÍTEZ-GUZMÁN A, ARRIAGA-PIZANO L, MORÁN J, et al. Endonuclease G takes part in AIF-mediated caspase-independent apoptosis in Mycobacterium bovis-infected bovine macrophages[J]. Vet Res, 2018, 49(1): 69-72. |
| [26] |
CHIPUK J E, KUWANA T, BOUCHIERHAYES L, et al. Direct activation of bax by p53 mediates mitoc; hondrial membrane permeabilization and apoptosis[J]. Science, 2004, 303(5660): 1010-1014. |
| [27] |
RAASCHOU NIELSEN O, ANDERSEN Z J, BEELEN R, et al. Air pollution and lung cancer incidence in 17 European cohorts:prospective analyses from the European Study of Cohorts for Air Pollution Effects(ESCAPE)[J]. Lancet Oncol, 2013, 14(9): 813-822. |
| [28] |
PAN Q, PAN H M, LOU H Z, et al. Inhibition of the angiogenesis and growth of Aloin in human colorectal cancer in vitro and in vivo[J]. Cancer Cell Int, 2013, 13(1): 69. |
| [29] |
ZHOU Y X, XIA W, YUE W, et al. Rhein:a review of pharmacological activities[J]. Evid Based Complement Alternat Med, 2015, 2015: 578107. |
| [30] |
CHEN Y Y, CHIANG S Y, LIN J G, et al. Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9[J]. Int J Oncol, 2010, 36(5): 1113-1120. |
 2020, Vol. 46
2020, Vol. 46


