扩展功能
文章信息
- 吴玉洁, 邢学农
- WU Yujie, XING Xuenong
- NIPA2通过JAK/STAT信号通路对高糖诱导的成骨细胞凋亡的调控作用及其机制
- Regulatory effect of NIPA2 on high glucose-induced osteoblast apoptosis via JAK/STAT signaling pathway and its mechanism
- 吉林大学学报(医学版), 2020, 46(02): 280-285
- Journal of Jilin University (Medicine Edition), 2020, 46(02): 280-285
- 10.13481/j.1671-587x.20200212
-
文章历史
- 收稿日期: 2019-06-20
糖尿病在世界范围内表现出越来越高的发病率,并且患者的年龄也日趋年轻化。随之而来的一系列糖尿病并发症(糖尿病肾病、糖尿病视网膜病变和糖尿病性骨质疏松症等)也越来越多。在患者发生糖尿病的基础上表现出骨质疏松的称之为糖尿病性骨质疏松症[1-2]。研究[3]显示:有30%以上的糖尿病患者会发生骨质疏松症,60%以上的糖尿病患者存在低骨量。因此,研究糖尿病相关骨代谢并用于指导临床治疗尤为重要。普瑞德-威利/安格曼综合征区域蛋白2(Prader-Willi/Angelman syndrome region protein 2, NIPA2)是一种镁转运蛋白,预测其有129个氨基酸和8个跨膜区域[4-5]。研究[6]显示:NIPA2与糖尿病的发病风险具有明显的相关性,但其具体作用机制研究甚少。Janus激酶/信号转导子与转录激活子(Janus kinase/signal transducer and activator of transcription, JAK/STAT)信号通路在细胞存活中具有重要的调控作用,参与很多疾病的发展,尤其是自身免疫疾病[7-9]。该通路在糖尿病肾病中的关键作用已有文献[10]报道,但是其在糖尿病性骨病中的作用尚不清楚。本研究采用高糖诱导的成骨细胞,检测细胞中NIPA2和JAK/STAT信号通路的表达,观察敲减NIPA2、抑制JAK/STAT信号通路对高糖诱导的成骨细胞增殖和凋亡的影响,阐明NIPA2的作用机制,旨在为糖尿病性骨病的治疗提供新的药物靶点。
1 材料与方法 1.1 细胞、主要试剂和仪器成骨细胞MC3T3-E1购自美国ATCC公司。α-MEM培养基、胎牛血清和噻唑蓝(methyl thiazolyl tetrazolium,MTT)和胰蛋白酶均购自美国Sellect公司,DMSO购自美国Sigma公司,AG490(用DMSO做溶剂)购自法国Gayman公司,LipofectamineTM2000、BCA蛋白定量试剂盒和逆转录试剂盒购自大连TaKaRa公司,PVDF膜购自德国罗氏诊断有限公司,SDS-PAGE试剂盒、ECL发光液和RIPA蛋白裂解液均购自碧云天生物技术公司,Annexin Ⅴ-FITC/PI凋亡检测试剂盒购自北京索莱宝公司。酶标仪购自美国Bio-Rad公司,流式细胞仪购自美国BD公司。
1.2 细胞培养将成骨细胞MC3T3-E1采用含10%胎牛血清的α-MEM培养基,置于37℃、5% CO2恒温培养箱中常规培养,待细胞生长至融合度约为75%时,用胰蛋白酶消化,进行传代。
1.3 细胞处理和分组将正常培养的MC3T3-E1细胞随机分为对照组、高糖(HG)组、HG+si-con组、HG+si-NIPA2组、HG+si-NIPA2+DMSO组和HG+si-NIPA2+AG490组。对照组:正常培养的MC3T3-E1细胞;HG组:将培养24 h的MC3T3-E1细胞采用26 nmol·L-1高糖处理24 h。将HG+si-con组、HG+si-NIPA2组、HG+ si-NIPA2+DMSO组和HG+si-NIPA2+AG490(50 μmol·L-1)组按照脂质体LipofectamineTM 2000说明书要求转染MC3T3-E1细胞,转染6 h后更换新鲜培养液继续培养48 h,然后采用26 nmol·L-1高糖处理24 h,采用qRT-PCR法检测转染效率。转染成功后,用于后续实验。
1.4 qRT-PCR法检测细胞中NIPA2 mRNA表达水平按照RNA抽提试剂盒说明书操作要求提取RNA,进行定量,然后按逆转录试剂盒说明书操作要求合成cDNA。最后按qRT-PCR试剂盒说明书操作要求进行NIPA2检测。以GAPDH为内参,采用2-ΔΔCt计算NIPA2 mRNA的表达水平。引物序列:NIPA2上游引物5′-CAGTTATGGTCATTCATGCTCCAA-3′,下游引物5′-TTAATATCAAGGCCACAATGACCAC-3′;GAPDH上游引物5′-TCCCTCAAGATTGCTAGCAA-3′, 下游引物5′-AGATCCACAACGGATACATT-3′。
1.5 Western blotting法检测细胞中NIPA2、P21、Cleaved caspase-3、p-JAK2和p-STAT3蛋白表达水平RIPA蛋白裂解液对细胞进行冰上蛋白裂解1 h,提取总蛋白,以BCA法测定样品蛋白的浓度。以4:1的比例加入蛋白上样缓冲液(×5),混匀,沸水浴变性10 min,离心取上清,进行SDS-PAGE蛋白电泳。然后,将蛋白湿转至PVDF膜,5%脱脂奶粉室温封闭1 h,TBST洗涤,4℃条件下,将封闭后的PVDF膜放入1: 1000倍稀释的一抗中反应过夜,以封闭液洗膜3次,每次5 min,再在37℃下将PVDF膜转入1:2000倍稀释的二抗中反应2 h。于暗室内ECL试剂盒显影曝光,以GAPDH为内参,以目的蛋白条带灰度值与GAPDH灰度值的比值表示目的蛋白的表达水平。
1.6 MTT法检测细胞增殖活性将细胞制成混悬液,调整密度至1×104mL-1,然后接种至96孔板,取适量“1.3”中各组细胞,每孔加20 μL MTT溶液(5 g·L-1),孵育4 h,终止培养,弃去培养液上清,然后每孔加150 μL DMSO,振荡使结晶充分融解,在490 nm波长处检测细胞吸光度(A)值。以A值表示细胞增殖活性。
1.7 Annexin Ⅴ-FITC/PI双染检测细胞凋亡采用500 μL Binding Buffer悬浮细胞,分别加入5 μLAnnexin Ⅴ-FITC避光反应20 min后再加入5 μLPI避光反应20 min,用300目铜筛过滤,最后在1 h内上流式细胞仪,计算细胞凋亡率。
1.8 统计学分析采用SPSS 21.0统计软件进行统计学分析。各组细胞中NIPA2 mRNA表达水平,细胞中NIPA2、P21、Cleaved caspase-3、p-JAK2和p-STAT3蛋白表达水平,细胞增殖活性和细胞凋亡率均以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用SNK-q检验,2组间样本均数比较采用两独立样本t检验。检验水准为α=0.05。
2 结果 2.1 2组成骨细胞中NIPA2 mRNA和蛋白表达水平与对照组比较,HG组细胞中NIPA2 mRNA和蛋白表达水平均明显降低(P < 0.01)。见图 1和表 1。
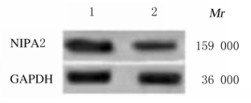
|
| Lane 1: Control group; Lane 2: HG group. 图 1 2组成骨细胞中蛋白表达电泳图 Fig. 1 Electrophoregram of expressions of NIPA2 protein in osteoblasts in two groups |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | NIPA2 mRNA | NIPA2 protein | |||||||||||||||||||||||||||
| Control | 1.00±0.03 | 1.00±0.06 | |||||||||||||||||||||||||||
| HG | 0.32±0.03* | 0.21±0.02* | |||||||||||||||||||||||||||
| t | 48.083 | 37.473 | |||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | |||||||||||||||||||||||||||
| *P < 0.01 compared with control group. | |||||||||||||||||||||||||||||
与HG+si-con组比较,HG+si-NIPA2组成骨细胞中NIPA2蛋白表达水平明显降低(P < 0.01),P21和Cleaved caspase-3蛋白表达水平明显升高(P < 0.01),在48和72 h时细胞增殖活性明显降低(P < 0.01),细胞凋亡率明显升高(P < 0.01)。见图 2、图 3和表 2。

|
| Lane1: HG+si-con group; Lane2: HG+si-NIPA2 group. 图 2 2组成骨细胞中NIPA2、P21和Cleaved caspase-3蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of NIPA2, P21, and Cleaved caspase-3 proteins in osteoblasts in varions groups |
|
|
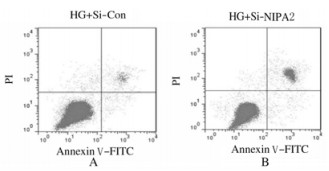
|
| A: HG+si-con group; B: HG+si-NIPA2 group. 图 3 流式细胞术检测2组成骨细胞凋亡率 Fig. 3 Apoptotic rates of osteoblasts in varions groups detected by flow cytometry |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | Protein expression level | Proliferation activity | Apoptotic rate (η/%) | ||||||||||||||||||||||||||
| NIPA2 | P21 | Cleaved caspase-3 | 24 h | 48 h | 72 h | ||||||||||||||||||||||||
| HG+si-con | 1.00±0.07 | 1.00±0.05 | 1.00±0.04 | 0.24±0.02 | 0.41±0.03 | 0.72±0.06 | 6.57±0.35 | ||||||||||||||||||||||
| HG+si-NIPA2 | 0.43±0.04* | 2.34±0.16* | 3.23±0.03* | 0.23±0.01 | 0.32±0.03* | 0.51±0.04* | 16.56±0.02* | ||||||||||||||||||||||
| t | 12.246 | 13.846 | 77.250 | 1.342 | 6.364 | 8.737 | 85.489 | ||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | 0.198 | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||
| *P < 0.01 compared with HG+si-con group. | |||||||||||||||||||||||||||||
与HG+si-con组比较,HG+si-NIPA2组成骨细胞中p-JAK2和p-STAT3蛋白表达水平均明显升高(P < 0.01)。见图 4和表 3。

|
| Lane1: HG+si-con group; Lane2: HG+si-NIPA2 group. 图 4 敲减NIPA2后2组成骨细胞中p-JAK2和p-STAT3蛋白表达电泳图 Fig. 4 Electrophoregram of expressions of p-JAK2 and p-STAT3 proteins in osteoblasts in two groups after knockdown of NIPA2 |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | Protein expression level | ||||||||||||||||||||||||||||
| p-JAK2 | p-STAT3 | ||||||||||||||||||||||||||||
| HG+si-con | 1.00±0.05 | 1.00±0.03 | |||||||||||||||||||||||||||
| HG+si-NIPA2 | 3.56±0.05* | 4.43±0.04* | |||||||||||||||||||||||||||
| t | 108.612 | 205.800 | |||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | |||||||||||||||||||||||||||
| *P < 0.01 compared with HG+si-con group. | |||||||||||||||||||||||||||||
与HG+si-NIPA2+DMSO组比较,HG+si-NIPA2+AG490组成骨细胞中NIPA2蛋白表达水平明显升高(P < 0.01),P21和Cleaved caspase-3蛋白表达水平明显降低(P < 0.01),在48和72 h时细胞增殖活性明显升高(P < 0.01),细胞凋亡率明显降低(P < 0.01)。见图 5和表 4。

|
| Lane1: HG+si-NIPA2 group; Lane2: HG+si-NIPA2+DMSO group; Lane3: HG+si-NIPA2+AG490 group. 图 5 抑制JAK/STAT信号通路后各组成骨细胞中NIPA2、P21和Cleaved caspase-3蛋白表达电泳图 Fig. 5 Electrophoregram of expressions of NIPA2, P21 and Cleaved caspase-3 proteins in osteoblasts in varions groups after inhibition of JAK/STAT signaling pathway |
|
|
| (n=9, x±s) | |||||||||||||||||||||||||||||
| Group | Protein expression level | Proliferation activity | Apoptotic rate (η/%) | ||||||||||||||||||||||||||
| NIPA2 | P21 | Cleaved caspase-3 | 24 h | 48 h | 72 h | ||||||||||||||||||||||||
| HG+si-NIPA2 | 0.99±0.05 | 1.00±0.03 | 1.00±0.06 | 0.23±0.02 | 0.29±0.01 | 0.49±0.03 | 17.12±1.02 | ||||||||||||||||||||||
| HG+si-NIPA2+DMSO | 1.00±0.08 | 1.01±0.07 | 0.99±0.04 | 0.22±0.02 | 0.30±0.01 | 0.50±0.05 | 16.94±1.00 | ||||||||||||||||||||||
| HG+si-NIPA2+AG490 | 1.36±0.08* | 0.44±0.04* | 0.23±0.02* | 0.21±0.01 | 0.50±0.03* | 0.83±0.04* | 9.28±0.02* | ||||||||||||||||||||||
| F | 78.412 | 388.338 | 940.661 | 3.000 | 344.455 | 202.140 | 264.985 | ||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | 0.069 | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||
| *P < 0.01 compared with HG+si-NIPA2+DMSO group. | |||||||||||||||||||||||||||||
NIPA2是一种高度选择性的镁离子转运蛋白,目前已发现其与2型糖尿病有关,但其具体作用机制尚未明确。CHAN等[6]在研究中发现NIPA1的异常与美国女性糖尿病风险具有密切的相关性。ZHAO等[11]研究发现:NIPA2在成骨细胞中的表达水平与晚期糖基化终末产物(advanced glycosylationend products, AGEs)呈剂量依赖性下调关系,且还可通过调节细胞内镁离子的含量进一步影响成骨细胞的成骨能力,调节成骨细胞的凋亡,提示NIPA2是治疗糖尿病骨质疏松症的潜在靶点,但NIPA2对糖尿病骨质疏松症的调控机制未做进一步研究。本研究采用qRT-PCR和Western blotting法检测高糖诱导MC3T3-E1细胞中NIPA2表达发现:NIPA2表达明显降低,再次证实了NIPA2在糖尿病性骨病中的表达下调;进一步研究通过敲减NIPA2后检测高糖诱导的MC3T3-E1细胞增殖和凋亡发现:敲减NIPA2可抑制其增殖,促进凋亡,并上调P21和Cleaved caspase-3的蛋白表达,为NIPA2治疗糖尿病骨病提供了更充分的理论依据。本研究结果显示:敲减NIPA2可上调JAK/STAT信号通路中p-JAK2和p-STAT3蛋白表达,为NIPA2的作用机制探究提供了新的依据。
JAK/STAT信号通路在机体内普遍存在,其参与细胞的增殖、分化和凋亡等基本活动以及免疫的调节和肿瘤的进展过程[12-17]。研究[18-19]显示:JAK/STAT信号通路在糖尿病肾病、糖尿病视网膜病变和糖尿病神经病变中均具有重要的作用,本文作者推测其在糖尿病性骨病中也具有一定的作用。LIU等[20]研究显示:JAK/STAT信号通路的失活在硫化氢(H2S)治疗糖尿病大鼠心肌纤维化中具有重要的作用,提示JAK/STAT信号通路在糖尿病心肌炎中具有调控作用。MIKAMI等[21]研究发现:碱性磷酸酶和地塞米松可通过抑制JAK/STAT信号通路增强骨的形成能力。本研究结果显示:在高糖诱导的成骨细胞中敲减NIPA2可明显增强JAK/STAT信号通路的活性,抑制JAK/STAT信号通路可逆转敲减NIPA2对高糖诱导成骨细胞的增殖抑制和凋亡促进作用,说明不仅敲减NIPA2可调控JAK/STAT信号通路的活性,相反,JAK/STAT信号通路也可反向作用于NIPA2的表达及功能,揭示了NIPA2在糖尿病性骨病中的作用机制与JAK/STAT信号通路的活性密切相关。
综上所述,敲减NIPA2可抑制高糖诱导的成骨细胞增殖,促进凋亡,其机制可能与激活JAK/STAT信号通路有关,本研究结果为NIPA2在糖尿病骨病中作用的研究奠定了基础。
| [1] |
KARAA A, GOLDSTEIN A. The spectrum of clinical presentation, diagnosis, and management of mitochondrial forms of diabetes[J]. Pediatr Diabetes, 2015, 16(1): 1-9. |
| [2] |
刘艳, 葛斌, 徐革, 等. 血清NGAL水平对2型糖尿病肾脏疾病的筛查价值[J]. 郑州大学学报(医学版), 2019, 54(3): 454-457. |
| [3] |
王国兴, 卢薇娜. 中老年患者中2型糖尿病和骨质疏松症的关系[J]. 全科医学临床与教育, 2017, 15(5): 544-547. |
| [4] |
GOYTAIN A, HINES R M, QUAMME G A. Functional characterization of NIPA2, a selective Mg2+ transporter[J]. Am J Physiol Cell Physiol, 2008, 295(4): 944-953. DOI:10.1152/ajpcell.00091.2008 |
| [5] |
CHEN C P, LIN S P, LEE C L, et al. Familial transmission of recurrent 15q11.2(BP1-BP2) microdeletion encompassing NIPA1, NIPA2, CYFIP1, and TUBGCP5 associated with phenotypic variability in developmental, speech, and motor delay[J]. Taiwan J Obstet Gynecol, 2017, 56(1): 93-97. DOI:10.1016/j.tjog.2016.12.002 |
| [6] |
CHAN K H, CHACKO S A, SONG Y, et al. Genetic variations in magnesium-related ion channels may affect diabetes risk among african american and hispanic american women[J]. J Nutr, 2015, 145(3): 418-424. |
| [7] |
田永贵, 张震, 尹婕, 等. 不同亚群CD8+T细胞表型、功能分子及分化相关基因的表达[J]. 郑州大学学报(医学版), 2019, 54(3): 318-323. |
| [8] |
AITTOMAKI S, PESU M. Therapeutic targeting of the JAK/STAT pathway[J]. Basic Clin Pharmacol Toxicol, 2014, 114(1): 18-23. DOI:10.1111/bcpt.12164 |
| [9] |
HEIM M H. The Jak-STAT pathway:cytokine signalling from the receptor to the nucleus[J]. J Recept Signal Transduct Res, 1999, 19(1-4): 75-120. DOI:10.3109/10799899909036638 |
| [10] |
陈宁, 郝军, 段惠军. 信号通路与糖尿病肾病肾小管细胞外基质沉积[J]. 河北医科大学学报, 2018, 39(2): 231-235. DOI:10.3969/j.issn.1007-3205.2018.02.027 |
| [11] |
ZHAO W, ZHANG W L, YANG B, et al. NIPA2 regulates osteoblast function via its effect on apoptosis pathways in type 2 diabetes osteoporosis[J]. Biochem Biophys Res Commun, 2019, 513(4): 883-890. DOI:10.1016/j.bbrc.2019.04.030 |
| [12] |
GRONER B, VON MANSTEIN V. Jak Stat signaling and cancer:Opportunities, benefits and side effects of targeted inhibition[J]. Mol Cell Endocrinol, 2017, 451(1): 1-14. |
| [13] |
GRIARDI T, VEREECKE S, SULIMA SO, et al. The T-cell leukemia-associated ribosomal RPL10 R98S mutation enhances JAK-STAT signaling[J]. Leukemia, 2018, 32(3): 809-819. DOI:10.1038/leu.2017.225 |
| [14] |
XU D, YIN C, WANG S, et al. JAK-STAT in lipid metabolism of adipocytes[J]. JAKSTAT, 2013, 2(4): e27203. |
| [15] |
NICOLAS C S, AMICI M, BORTOLOTTO Z A, et al. The role of JAK-STAT signaling within the CNS[J]. JAKSTAT, 2013, 2(1): e22925. |
| [16] |
ZHANG Y, ZHANG L, ZUO Q, et al. JAK-STAT signaling regulation of chicken embryonic stem cell differentiation into male germ cells[J]. In Vitro Cell Dev Biol-Anim, 2017, 53(8): 728-743. DOI:10.1007/s11626-017-0167-9 |
| [17] |
SILVER M L, LI WX. JAK-STAT in heterochromatin and genome stability[J]. JAKSTAT, 2013, 2(3): e26090. |
| [18] |
董志军, 陈志宏, 陶相宜, 等. 丝胶通过调节JNK信号通路抑制糖尿病大鼠视网膜神经细胞凋亡[J]. 河北医学, 2018, 24(5): 794-797. DOI:10.3969/j.issn.1006-6233.2018.05.024 |
| [19] |
MARRERO MB, BANES-BERCELIAK, STERN DM, et al. Role of the JAK/STAT signaling pathway in diabetic nephropathy[J]. Am J Physiol Renal Physiol, 2006, 290(4): F762-F768. DOI:10.1152/ajprenal.00181.2005 |
| [20] |
LIU M J, LI Y, LIANG B, et al. Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway[J]. Int J Mol Med, 2018, 41(4): 1867-1876. |
| [21] |
MIKAMI Y, ASANO M, HONDA M J, et al. Bone morphogenetic protein 2 and dexamethasone synergistically increase alkaline phosphatase levels through JAK/STAT signaling in C3H10T1/2 cells[J]. J Cell Physiol, 2010, 223(1): 123-133. DOI:10.1002/jcp.22017 |
 2020, Vol. 46
2020, Vol. 46


