扩展功能
文章信息
- 徐建国, 曹洪涛, 张子龙, 冀保妍, 王春秋, 李生栋, 刘国庆
- XU Jianguo, CAO Hongtao, ZHANG Zilong, JI Baoyan, WANG Chunqiu, LI Shengdong, LIU Guoqing
- LincRNA-p21敲减对胃癌细胞生长和转移的影响及其作用机制
- Effects of LincRNA-p21 knockdown on growth and metastasis of gastric cancer cells and their mechanisms
- 吉林大学学报(医学版), 2020, 46(02): 266-273
- Journal of Jilin University (Medicine Edition), 2020, 46(02): 266-273
- 10.13481/j.1671-587x.20200210
-
文章历史
- 收稿日期: 2019-04-08
2. 青海省人民医院肿瘤内科, 青海 西宁 810001;
3. 青海省人民医院消化内科, 青海 西宁 810001
2. Department of Oncology, Qinghai Provincial People's Hospital, Xi-ning 810001, China;
3. Department of Gastroenterology, Qinghai Provincial People's Hospital, Xi-ning 810001, China
胃癌是亚洲最常见的消化道肿瘤之一,在导致死亡的癌症类型中位居第3位[1],其死亡率在国内也有增高趋势[2-3]。因此,确定新的分子标志物以诊断早期胃癌是一个迫切需要解决的问题,且寻找的生物标志物不仅能诊断早期胃癌,也能作为治疗胃癌的分子靶点。
长链非编码RNA(long noncoding RNA,LncRNA)是一种长度超过200个核酸序列的转录因子,近年来被广泛关注。而LincRNA-p21被认为是p53的转录调节因子,在p53调控网络起着“效应器”的作用,可抑制p53的转录,调节细胞周期与凋亡[4-5]。有研究[6]表明LincRNA-p21与前列腺癌的发生及发展有关,但其在胃癌发生过程中的作用尚不清楚。Janus激酶/信号转导子与转录激活子(Janus kinase/signal transducer and activator of transcription,JAK/STAT)信号通路被认为是与肿瘤的发生、迁移和侵袭等过程相关的信号通路,其激活可导致STAT5蛋白异常磷酸化,p-STAT5转移至细胞核结合在DNA-STAT5结合位点上,加速细胞增殖基因的转录,从而导致肿瘤的发生[7]。近年来,有研究[8-9]表明多种LncRNA参与JAK/STAT信号通路的调节。
为了探讨LincRNA-p21是否通过此通路来调节肿瘤的发生发展、迁移及侵袭,本文作者首先研究LincRNA-p21在胃癌组织和多种胃癌细胞中的表达情况,再利用体外及体内实验,采用慢病毒感染方法敲减胃癌细胞MGC-803中LincRNA-p21表达,观察LincRNA-p21敲减对MGC-803细胞的生长、迁移及侵袭功能的影响,且研究其对JAK-STAT信号通路的调节作用,来阐明LincRNA-p21低表达可能是胃癌进展的关键事件之一。
1 材料与方法 1.1 实验动物、细胞、主要试剂和仪器8只6周龄SPF级雄性BALB/c裸鼠购自北京维通利华实验动物技术有限公司,动物合格证号:SCXK(京)2016-0011。胃癌细胞MGC-803、MKN-45、SGC-790和正常胃黏膜细胞GES-1(ATCC细胞库,美国)。5-乙炔基-2′-脱氧尿苷(EdU)检测试剂盒(广州锐博生物科技有限公司),胎牛血清和DMEM培养基(Gibco公司, 美国),CCK-8细胞计数试剂盒(武汉默沙克生物科技有限公司),RNAiso Plus试剂盒、逆转录试剂盒和SuperReal PreMix试剂盒[天根生化科技(北京)有限公司],RIPA裂解液、BCA蛋白定量试剂盒和β-actin抗体(上海碧云天生物科技有限公司),p-JAK1抗体、p-STAT3抗体和pSTAT5抗体(CST公司, 美国),兔抗鼠二抗[艾博抗(上海)贸易有限公司],靶向抑制LincRNA-p21的慢病毒载体(sh-LincRNA-p21)和对照(sh-NC)由上海吉玛公司合成。BX3型荧光显微镜(Olympus公司,日本),ABI 7500荧光定量PCR仪(Applied Biosystems公司, 美国),iMark多功能酶标仪(Bio-Rad公司,美国)。
1.2 组织来源收集2014—2016年青海省人民医院肿瘤外科留存的20例Ⅲ期(T分期)胃癌患者行肿瘤切除术的胃癌肿瘤组织和配对的癌旁对照组织(距离肿瘤组织边缘2.5 cm)。本研究通过青海省人民医院伦理委员会批准(No.20140315116)。
1.3 细胞培养胃癌细胞MGC-803、MKN-45、SGC-790与正常胃黏膜上皮细胞GES-1培养于含10%胎牛血清的DMEM培养基、37℃的培养箱(5%CO2,95%湿度)中培养,当细胞达到90%融合时按照1:3传代。取对数期细胞用于后续实验。
1.4 细胞处理和分组将2×105个MGC-803细胞接种至24孔板中,待细胞贴壁后,吸弃培养基,加入2 mL含有6 mg·L-1聚凝胺的新鲜培养基,并分别加入感染复数(multiplicity of infection,MOI)为40的sh-LincRNA-p21和sh-NC病毒液,继续培养24 h后,更换新鲜的完全培养基,再继续培养24 h。该病毒感染的MGC-803细胞经RT-qPCR法检测干扰效果后,收集细胞,分别命名为sh-NC组和sh-LincRNA-p21组。将密度为1×106mL-1且稳定感染sh-LincRNA-p21的MGC-803细胞接种于6孔板,待细胞贴壁后,采用10 μg·L-1 JAK-STAT通路特异性抑制剂AG490处理细胞30 min。收集细胞并命名为AG490+sh-LincRNA-p21组。
1.5 RT-qPCR法检测LincRNA-p21 mRNA表达水平收集胃癌肿瘤组织、癌旁组织、MGC-803细胞、MKN-45细胞、SGC-790细胞和GES-1细胞与sh-NC组及sh-LincRNA-p21组MGC-803细胞分别置于EP管中,按照RNAiso Plus说明书步骤,依次采用RNAiso Plus变性缓冲液、20%体积氯仿、异丙醇、75%乙醇萃取细胞总RNA。其次,取1 μg总RNA通过逆转录试剂盒方法逆转录合成cDNA。再次取cDNA模板和目的基因引物,按照SuperReal PreMix试剂盒说明书利用ABI 7500荧光定量PCR仪进行RT-qPCR。反应条件:95℃、30s,然后进行40循环(95℃、2s,60℃、30s,66℃、1min)。选用GAPDH作为目的基因的内参,目的基因的相对表达水平以2-ΔΔCt表示。引物序列:LincRNA-p21正向引物5′-TGTAGTTTTCGGAGTTAGTGTCGCGC-3′,反向引物5′-CCTACGATCGAAAACGACGCGAACG-3′;GAPDH正向引物5′-GGGAAATTCAACGGCACAGT-3′,反向引物5′-AGATGGTGATGGGCTGCCC-3′。
1.6 EdU掺入法检测细胞EdU掺入百分比将密度为1×106 mL-1的sh-NC组和sh-LincRNA-p21组MGC-803细胞铺于96孔板上,待细胞贴壁后,培养48 h,按照EdU检测试剂盒操作步骤,加入100 mg·L-1EdU试剂,孵育2 h,DAPI试剂染核5 min。荧光显微镜下采用随机视野法拍摄5张细胞图像并计数。每组实验重复4次。EdU掺入百分比=EdU+DAPI+细胞数/DAPI细胞数×100%。
1.7 CCK-8法检测细胞活性将密度为1×106 mL-1的sh-NC组、sh-LincRNA-p21组和AG490+sh-LincRNA-p21组MGC-803细胞铺于96孔板上,待细胞贴壁后,培养24、48和72 h后,按照CCK-8细胞计数试剂盒操作步骤,加入10 μL CCK-8试剂,孵育2 h。酶标仪检测450 nm波长处的吸光度(A)值。每组实验重复4次,取A值的平均值,并绘制细胞生长曲线。细胞活性以A值的平均值表示。
1.8 Transwell法检测细胞迁移数和细胞侵袭数将sh-NC组、sh-LincRNA-p21组和AG490+sh-LincRNA-p21组MGC-803细胞采用无血清培养基分别制备成浓度为5×105 mL-1的单细胞悬液。细胞迁移检测:在Transwell上室加入100 μL单细胞悬液,下室加入600 μL含有10%胎牛血清的DMEM培养48 h,取出上室,擦除上室上膜细胞,0.25%的结晶紫染色上室下膜细胞10 min,显微镜下采用随机视野法拍摄5张细胞图像并计数。细胞侵袭检测:先将Transwell上室铺10%的基底膜,其余操作步骤与细胞迁移步骤相同。上述每组实验均重复4次。
1.9 Western blotting法检测各组细胞中p-JAK1、p-STAT3和p-STAT5蛋白表达水平收集sh-NC组和sh-LincRNA-p21组MGC-803细胞,利用RIPA裂解液萃取蛋白。利用SDS-聚丙烯酰胺凝胶电泳分离蛋白。分离的蛋白经转膜和封闭后,在室温条件下以1:1000的稀释比例孵育一抗(p-JAK1、p-STAT3、p-STAT5和GAPDH) 2 h。TBST洗涤后,室温条件下孵育HRP标记的二抗1 h。TBST洗涤后利用化学发光成像仪显影。各组细胞中p-JAK1、p-STAT3和p-STAT5蛋白条带采用Image J软件扫描灰度值,并以GAPDH灰度值进行量化。目的蛋白表达水平=目的蛋白条带灰度值/GAPDH灰度值×100%。
1.10 体内成瘤实验裸鼠饲养于恒定室温25℃、45%~55%相对湿度的青海省地方病预防控制所SPF动物房,每天光照12 h,常规饲料饲养1周后进行实验。分别将1×107个稳定感染sh-LincRNA-p21和sh-NC的MGC-803细胞移植入裸鼠颈部皮下成瘤,命名为sh-LincRNA-p21组和sh-NC组,每组4只裸鼠。定期用游标卡尺测量肿瘤大小,瘤体体积按照1/2×长×宽2公式近似估算。饲养30 d后取肿瘤称质量。
1.11 统计学分析采用Graphpad Prism 7.0软件对数据进行统计学分析。LincRNA-p21相对表达水平、EdU掺入百分比、细胞活性、细胞迁移数、细胞侵袭数、瘤体体积、瘤体质量以及p-JAK1、p-STAT3、p-STAT5蛋白相对表达水平均以x±s表示,2组间样本均数比较采用两独立样本t检验,多组间样本均数比较采用单因素方差分析。以P < 0.05为差异有统计学意义。
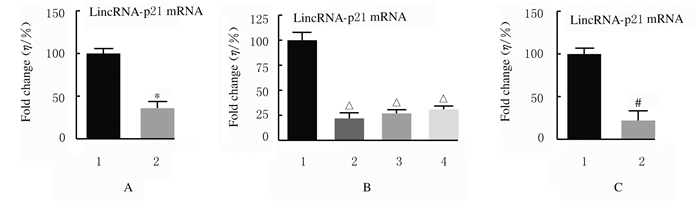
2 结果 2.1 LincRNA-p21mRNA在胃癌组织和不同胃癌细胞中的表达水平与癌旁组织比较,胃癌组织中LincRNA-p21 mRNA表达水平明显降低(P < 0.01)。与正常胃黏膜上皮细胞GES-1比较,胃癌细胞MGC-803、MKN-45和SGC-790中LincRNA-p21 mRNA表达水平均明显降低(P < 0.01)。与sh-NC组比较,sh-LincRNA-p21组MGC-803细胞中LincRNA-p21 mRNA表达水平明显降低(P < 0.01)。见图 1。
|
| A:n=20, *P < 0.01 vs adjacent tissue; B:n=4, △P < 0.01 vs GES-1 cells; C:n=4, #P < 0.01 vs sh-NC group. A: Expression levels of LincRNA-p21 mRNA in gastric cancer tissue and adjacent tissue; 1:Adjacent; 2:Tumor.B: Expression levels of LincRNA-p21 mRNA in GES-1, MGC-803, MKN-45 and SGC-790 cells; 1:GES-2 cells; MGC-803 cells; 3:MKN-45 cells; 4:SGC-790 cells; C: Expression levels of sh-LincRNA-p21 in sh-NC group and sh-Linc RNA-p21 group; 1:sh-NC group; 2:sh-LincRNA-p21 group. 图 1 胃癌组织和不同胃癌细胞中LincRNA-p21mRNA表达水平 Fig. 1 Expression levels of LincRNA-p21 mRNA in gastric cancer tissue and different gastric cancer cells |
|
|
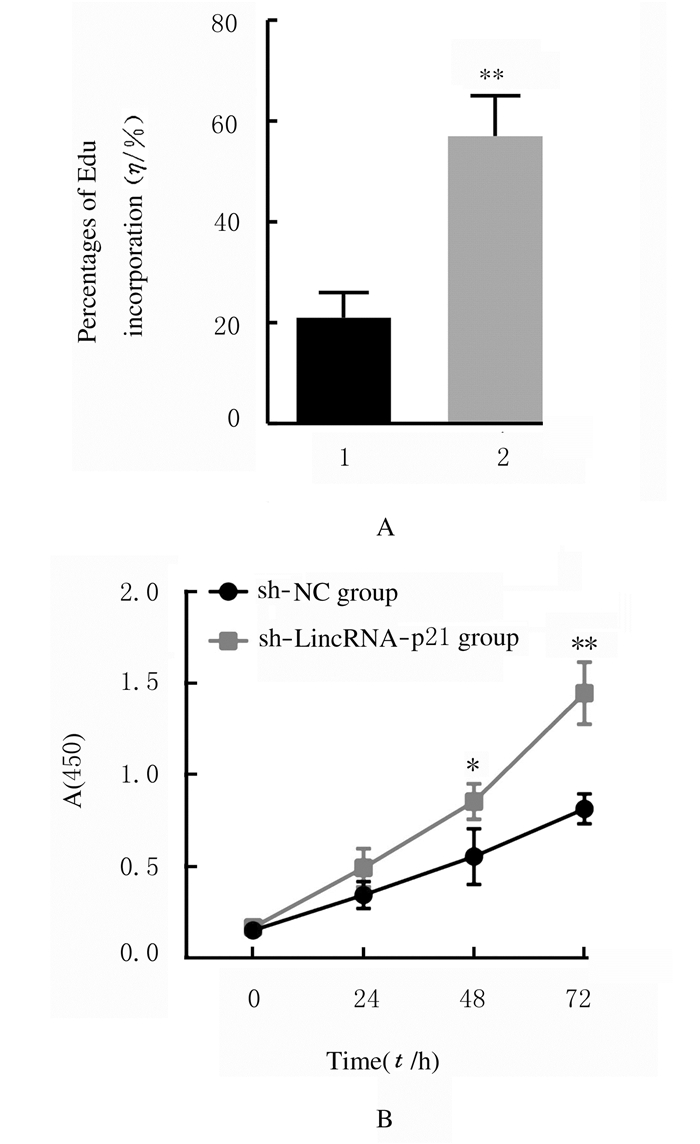
与sh-NC组比较,sh-LincRNA-p21组MGC-803细胞中EdU掺入百分比明显升高(P < 0.01)。与sh-NC组比较,sh-LincRNA-p21组MGC-803细胞活性升高,转染后48和72 h时,细胞活性明显升高(P < 0.05或P < 0.01)。见图 2(插页三)和图 3。

|
| A-C:sh-NC group; D-F:sh-LincRNA-p21 group; A, D:EdU; B, E:DAPI; C, F:Merge. 图 2 sh-NC组和sh-LincRNA-p21组MGC-803细胞EdU染色图像(×200) Fig. 2 EdU staining images of MGC-803 cells in sh-NC group and sh-LincRNA-p21 group (×200) |
|
|
|
| n=4, *P < 0.05, * *P < 0.01 vs sh-NC group.1:sh-NC group; 2:sh-LincRNA-p21 group. 图 3 sh-NC组和sh-LincRNA-p21组MGC-803细胞的EdU掺入百分比(A)和细胞活性(B) Fig. 3 Percentages of EdU incorporation (A) and cell viabilities (B) of MGC-803 cells in sh-NC group and sh-LincRNA-p21 group |
|
|
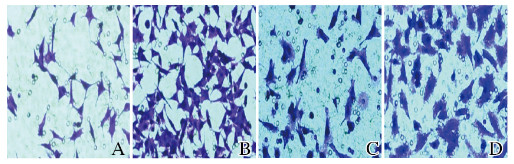
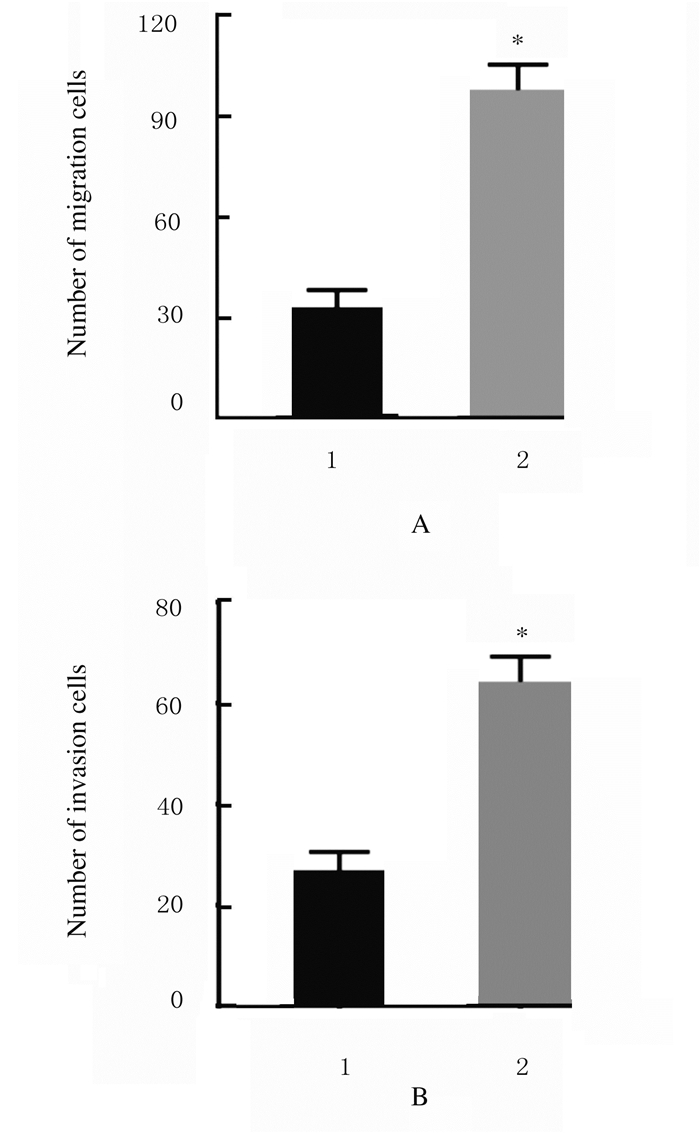
与sh-NC组比较,sh-LincRNA-p21组MGC-803细胞迁移数和侵袭数均明显增加(P < 0.01)。见图 4(插页三)和图 5。
|
| A, C:sh-NC group; B, D:sh-LincRNA-p21 group. 图 4 sh-NC组和sh-LincRNA-p21组MGC-803细胞迁移(A, B)和侵袭(C, D)图像(结晶紫,×400) Fig. 4 Migration(A, B) and invasion(C, D) images of MGC-803 cells in sh-NC group and sh-LincRNA-p21 group (Crystal violet, ×400) |
|
|
|
| n=4, * *P < 0.01 vs sh-NC group.1:sh-NC group; 2:sh-LincRNA-p21 group. 图 5 sh-NC组和sh-LincRNA-p21组中MGC-803细胞迁移数(A)和侵袭数(B) Fig. 5 Number of migration (A) and invasion (B) of MGC-803 cells in sh-NC group and sh-LincRNA-p21 group |
|
|
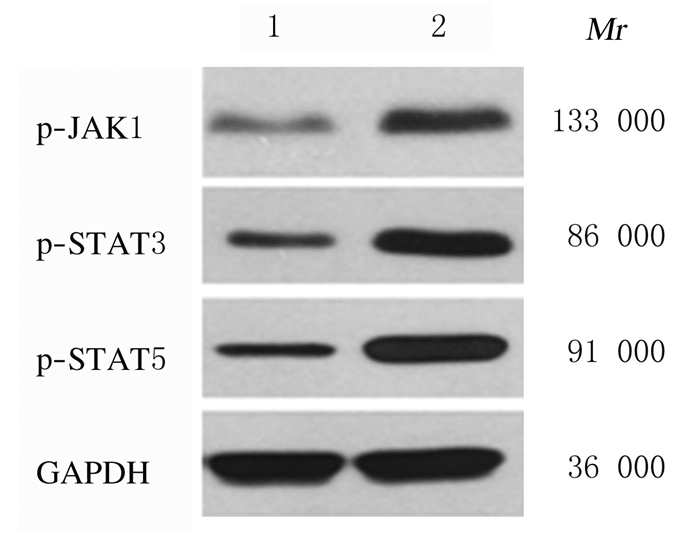
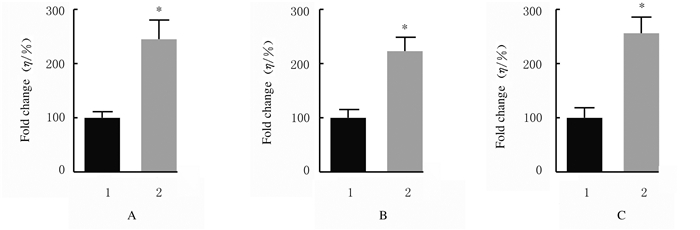
与sh-NC组比较,sh-LincRNA-p21组MGC-803细胞中p-JAK1、p-STAT3和p-STAT5蛋白相对表达水平均明显升高(P < 0.01)。见图 6和图 7。
|
| Lane 1: sh-NC group; Lane 2: sh-LincRNA-p21 group. 图 6 sh-NC组和sh-LincRNA-p21组MGC-803细胞中p-JAK1、p-STAT3和p-STAT5蛋白表达电泳图 Fig. 6 Electrophoregram of expressions of p-JAK1, p-STAT3, and p-STAT5 proteins in MGC-803 cells in sh-NC group and sh-LincRNA-p21 group |
|
|
|
| n=4, *P < 0.01 vs sh-NC group. A: p-JAK1; B: p-STAT3; C: p-STAT5;1:sh-NC group; 2:sh-LincRNA-p21 group. 图 7 sh-NC组和sh-LincRNA-p21组MGC-803细胞中p-JAK1、p-STAT3和p-STAT5蛋白表达水平 Fig. 7 Expression levels of p-JAK1, p-STAT3, and p-STAT5 proteins in MGC-803 cells in sh-NC group and sh-LincRNA-p21 group |
|
|
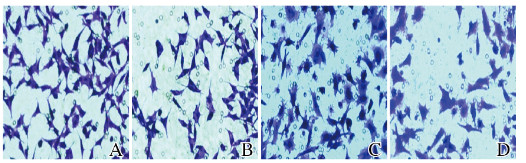
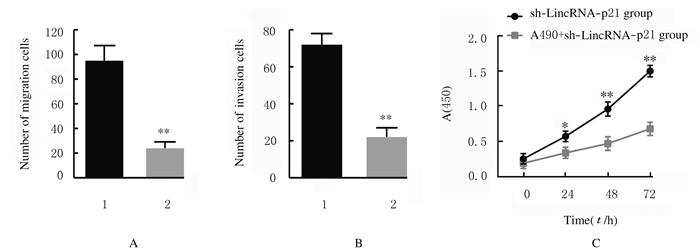
与sh-LincRNA-p21组比较,AG490+sh-LincRNA-p21组MGC-803细胞活性在24、48和72 h时均明显降低(P < 0.05或P < 0.01)。与sh-LincRNA-p21组比较,AG490+sh-LincRNA-p21组MGC-803细胞迁移数与侵袭数明显降低(P < 0.01)。见图 8(插页三)和图 9。
|
| A, C:sh-LincRNA-p21;B, D:AG490+sh-LincRNA-p21 group. 图 8 sh-LincRNA-p21组和AG490+sh-LincRNA-p21组MGC-803细胞迁移(A, B)和侵袭(C, D)图像(结晶紫,×400) Fig. 8 Migration(A, B) and invasion(C, D) images of MGC-803 cells in sh-LincRNA-p21 group and AG490+sh-LincRNA-p21 group (Crystal violet, ×400) |
|
|
|
| n=4, *P < 0.05, * *P < 0.01 vs sh-LincRNA-p21 group.1:sh-LincRNA-p21 group; 2:AG490+sh-LincRNA-p21group. 图 9 sh-LincRNA-p21组和AG490+sh-LincRNA-p21组MGC-803细胞迁移数(A)、侵袭数(B)和细胞活性(C) Fig. 9 Number of migration (A) and invasion (B) and viabilities (C) of MGC-803 cells in sh-LincRNA-p21 group and AG490+sh-LincRNA-p21 group |
|
|
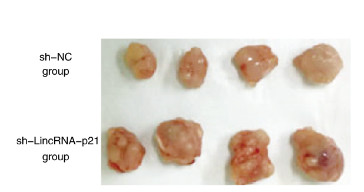
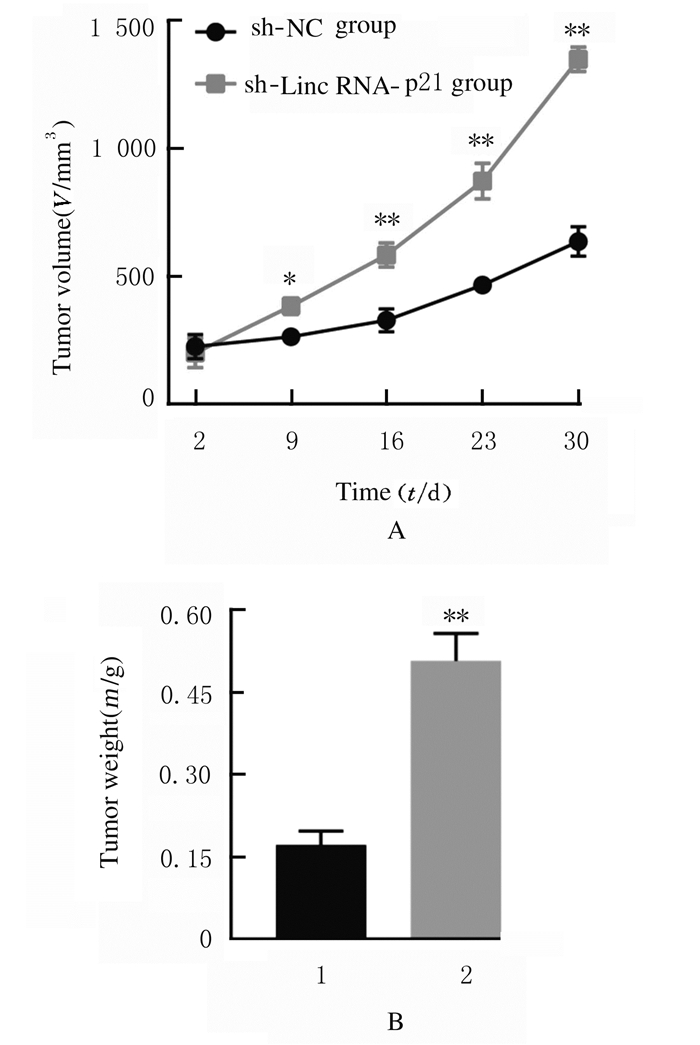
与sh-NC组比较,sh-LincRNA-p21组在移植后第9、16、23和30天时瘤体体积明显增加(P < 0.05或P < 0.01),第30天时瘤体质量也明显增加(P < 0.01)。见图 10(插页三)和图 11。
|
| 图 10 第30天时sh-NC组和sh-LincRNA-p21组荷瘤裸鼠瘤体大体形态 Fig. 10 Gross morphology of tumors of tumor-bearing nude mice in sh-NC group and sh-LincRNA-p21 group |
|
|
|
| n=4, *P < 0.05, **P < 0.01 vs sh-NC group.1:sh-NC group; 2:sh-LincRNA-p21 group. 图 11 sh-NC组和sh-LincRNA-p21组瘤体体积(A)和质量(B) Fig. 11 Volumes (A) and weights (B) of tumor in sh-NC group and sh-LincRNA-p21 group |
|
|
胃癌的高发生率和低生存率对人类健康构成严重威胁,且其发展是一个多步骤的过程,涉及遗传改变及表观遗传等多种因素[10]。因此,了解胃癌的发生机制对于发现新的治疗靶点有相当重要的意义。LncRNAs在癌症发展过程中的作用被众多学者所关注,主要是因为其可作为信号、向导等调节细胞内的病理生理过程[11-13]。而LincRNA-p21是一种可抑癌的非编码RNA,其主要是作用于p53基因调节细胞周期和凋亡[14]。基于LincRNA-p21表达特征,本文作者发现其在3种胃癌细胞系中表达水平较正常胃黏膜上皮细胞GES-1均下调。另外,临床病理相关研究[15]表明:LincRNA-p21的低表达水平与肿瘤高侵袭性、高转移性风险和更广泛的TNM转移分期有关;提示LincRNA-p21低表达可能与胃癌进展有关。为证明这一猜测,本研究采用sh-LincRNA-p21感染胃癌MGC-803细胞,结果发现LincRNA-p21敲减可促进MGC-803细胞增殖、迁移和侵袭性。而体内成瘤实验同样证明了LincRNA-p21敲减可促进移植瘤的生长。
异常激活JAK-STAT信号可促进包含胃癌在内的多种肿瘤细胞增殖、迁移、侵袭及上皮间质转化(epithelial-mesenchymal transition,EMT)[16-18]。研究[19-20]显示:LncRNA可作为JAK-STAT信号通路的重要调节因子来调节肿瘤的生长与分化。因此,本研究选取了与肿瘤发生相关的JAK/STAT信号通路作为研究重点,用来探讨LincRNA-p21低表达对胃癌细胞增殖、迁移和侵袭的促进作用。本研究结果显示:LincRNA-p21敲减能促进MGC-803细胞JAK/STAT信号通路关键分子p-JAK1、p-STAT3与p-STAT5的表达。
本研究利用JAK-STAT通路特异性抑制剂AG490来验证其对感染sh-LincRNA-p21的MGC-803细胞生长、迁移和侵袭的影响,结果显示:AG490能逆转sh-LincRNA-p21对细胞生长、迁移及侵袭的促进能力,提示LincRNA-p21敲减对胃癌细胞生长与转移的促进作用可能与其促进JAK/STAT通路信号活性有关。
综上所述,LincRNA-p21敲减可促进胃癌细胞生长、迁移与侵袭。而这种作用可能与促进JAK/STAT信号通路活性有关联,但其激活此通路的具体分子机制尚待更深入的研究。另外,本研究结果提示LincRNA-p21低表达可能是胃癌进展的潜在危险因素之一。
| [1] |
郑振东, 韩涛. 胃癌诊疗研究进展[J]. 临床军医杂志, 2017, 45(1): 1-4. |
| [2] |
左婷婷, 郑荣寿, 曾红梅, 等. 中国胃癌流行病学现状[J]. 中国肿瘤临床, 2017, 44(1): 52-58. DOI:10.3969/j.issn.1000-8179.2017.01.881 |
| [3] |
TAHARA T, ARISAWA T. DNA methylation as a molecular biomarker in gastric cancer[J]. Epigenomics, 2015, 7(3): 475-486. DOI:10.2217/epi.15.4 |
| [4] |
WU G Z, CAI J, HAN Y, et al. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity[J]. Circulation, 2014, 130(17): 1452-1465. DOI:10.1161/CIRCULATIONAHA.114.011675 |
| [5] |
徐艳雪, 朱友明, 陈乔尔. p53相关长链非编码RNA在肿瘤发生发展中作用的研究[J]. 安徽医药, 2018, 22(7): 1230-1235. DOI:10.3969/j.issn.1009-6469.2018.07.004 |
| [6] |
WANG X H, RUAN Y, WANG X J, et al. Long intragenic non-coding RNA lincRNA-p21 suppresses development of human prostate cancer[J]. Cell Prolif, 2017, 50(2): e12318. DOI:10.1111/cpr.12318 |
| [7] |
于景霞, 刘婷, 李沁恺, 等. 特异性抑制JAK/STAT3信号通路对大鼠肝细胞癌的影响[J]. 胃肠病学, 2017, 22(5): 272-275. DOI:10.3969/j.issn.1008-7125.2017.05.004 |
| [8] |
ZHANG W Y, DONG R, DIAO S, et al. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells[J]. Stem Cell Res Ther, 2017, 8(1): 30. DOI:10.1186/s13287-017-0485-6 |
| [9] |
朱银银, 艾合麦提江·库尔班, 沈钲杰, 等. 胃癌中长非编码RNA调节网络的研究进展[J]. 中国细胞生物学学报, 2017, 39(10): 1369-1373. DOI:10.11844/cjcb.2017.10.0142 |
| [10] |
章华丽, 蔡希, 赵素凡, 等. 非胃肠道表现为首发症状的胃癌危险因素及预后分析[J]. 中国预防医学杂志, 2018, 19(6): 432-436. |
| [11] |
田智, 刘亮华, 廖江涛. 长链非编码核糖核酸Malat-1、p21和GAS5在大肠癌组织中的表达及其诊断价值[J]. 华中科技大学学报(医学版), 2017, 46(1): 10-14. |
| [12] |
LU M Y, LIAO Y W, CHEN P Y, et al. Targeting LncRNA HOTAIR suppresses cancer stemness and metastasis in oral carcinomas stem cells through modulation of EMT[J]. Oncotarget, 2017, 8(58): 98542-98552. |
| [13] |
PAN Y Y, PAN Y Q, CHENG Y, et al. Knockdown of LncRNA MAPT-AS1 inhibites proliferation and migration and sensitizes cancer cells to paclitaxel by regulating MAPT expression in ER-negative breast cancers[J]. Cell Biosci, 2018, 8: 7. DOI:10.1186/s13578-018-0207-5 |
| [14] |
SHI J G, ZHANG W, TIAN H Y, et al. lncRNA ROR promotes the proliferation of renal cancer and is negatively associated with favorable prognosis[J]. Mol Med Rep, 2017, 16(6): 9561-9566. DOI:10.3892/mmr.2017.7775 |
| [15] |
SONG P, JIANG B, LIU Z J, et al. A three-lncRNA expression signature associated with the prognosis of gastric cancer patients[J]. Cancer Med, 2017, 6(6): 1154-1164. DOI:10.1002/cam4.1047 |
| [16] |
PENCIK J, PHAM H T, SCHMOELLERL J, et al. JAK-STAT signaling in cancer:from cytokines to non-coding genome[J]. Cytokine, 2016, 87: 26-36. DOI:10.1016/j.cyto.2016.06.017 |
| [17] |
KHANNA P, CHUA P J, BAY B H, et al. The JAK/STAT signaling cascade in gastric carcinoma (Review)[J]. Int J Oncol, 2015, 47(5): 1617-1626. DOI:10.3892/ijo.2015.3160 |
| [18] |
BI C L, ZHANG Y Q, LI B, et al. MicroRNA-520a-3p suppresses epithelial-mesenchymal transition, invasion, and migration of papillary thyroid carcinoma cells via the JAK1-mediated JAK/STAT signaling pathway[J]. J Cell Physiol, 2019, 234(4): 4054-4067. DOI:10.1002/jcp.27199 |
| [19] |
OLSON T L, LOUGHRAN T P Jr. Restoring the long noncoding RNA MEG3 indicates a potential role for JAK-STAT signaling in chronic myeloid leukemia[J]. EBioMedicine, 2018, 35: 24. DOI:10.1016/j.ebiom.2018.08.019 |
| [20] |
GONG W, QIE S, HUANG P, et al. Deletion of long noncoding RNA EFNA3 aggravates hypoxia-induced injury in PC-12 cells by upregulation of miR-101a[J]. J Cell Biochem, 2019, 120(1): 836-847. DOI:10.1002/jcb.27444 |
 2020, Vol. 46
2020, Vol. 46


