扩展功能
文章信息
- 王雪冬, 王迎迎, 刘索宁, 王靖, 刘昊鹏, 刘楠, 裴爱月, 秦晶, 冯春生, 朴美花
- WANG Xuedong, WANG Yingying, LIU Suoning, WANG Jing, LIU Haopeng, LIU Nan, PEI Aiyue, QIN Jing, FENG Chunsheng, PIAO Meihua
- 七氟醚对HT22小鼠海马神经元细胞DNA的损伤及其机制
- DNA damage of HT22 mouse hippocampal neuronal cells induced by sevoflurane and its mechanism
- 吉林大学学报(医学版), 2020, 46(02): 240-247
- Journal of Jilin University (Medicine Edition), 2020, 46(02): 240-247
- 10.13481/j.1671-587x.20200206
-
文章历史
- 收稿日期: 2019-09-03
2. 吉林大学第一医院胃结直肠外科, 吉林 长春 130021;
3. 吉林大学第一医院内镜中心, 吉林 长春 130021;
1. Department of Anesthesiology, First Hospital, Jilin University, Changchun 130021, China
2. Department of Gastrointestinal Surgery, First Hospital, Jilin University, Changchun 130021, China;
3. Clinical Medical College, Jilin University, Changchun 130021, China;
1. Department of Endoscope Center, First Hospital, Jilin University, Changchun 130021, China
吸入麻醉药七氟醚具有血气分配系数低、血流动力学稳定和苏醒快等优点,被广泛应用于外科手术麻醉。但近年来研究[1]发现:发育中大脑暴露于七氟醚中会导致广泛的神经元凋亡和神经退行性改变,进而导致远期的学习记忆、认知障碍和行为异常。已有研究[2-4]显示:七氟醚能够对未成熟脑产生神经毒性作用,进而诱发长期的脑功能发育障碍,但其机制尚不完全清楚。有研究[5]表明七氟醚通过影响新生大鼠大脑中的代谢途径,从而产生脑神经毒性作用。也有研究[6]表明氧化酶抑制剂可减少七氟醚导致的新生鼠长期记忆功能损害。DNA是生物的主要遗传物质,其完整性的维系对生命个体生存和维持物种稳定起至关重要的作用。研究[7]显示:DNA损伤修复基因的敲除能够导致小鼠胚胎神经发育异常和神经元细胞死亡,进而诱发长期脑功能障碍。研究[8]证实吸入麻醉药异氟醚能够通过诱导DNA损伤引起老年小鼠脑神经损伤及加重神经退行性改变。但七氟醚的神经毒性作用是否与DNA损伤有关尚无明确报道。因此,本研究以HT22小鼠海马神经元细胞为研究对象,观察七氟醚对海马神经元细胞DNA损伤的影响,阐明其诱导脑神经毒性作用的可能机制。
1 材料与方法 1.1 细胞、主要试剂和仪器HT22小鼠海马神经元细胞购于上海生物科技有限公司。DMEM高糖培养液、胎牛血清、青霉素、链霉素、多聚赖氨酸、0.25%胰蛋白酶和磷酸缓冲液(PBS)均购于美国Gibco公司,四甲基偶氮唑盐(MTT)和抗氧化剂N-乙酰半胱氨酸(NAC)购自美国Sigma公司,乳酸脱氢酶(LDH)细胞毒性检测试剂盒和DCFH-DA活性氧检测试剂盒购于上海碧云天生物技术有限公司,七氟醚购于艾伯维医药贸易(上海)有限公司,BCA蛋白定量试剂盒购于美国Pierce公司,小鼠抗Histone蛋白购于美国Proteintech公司,小鼠抗毛细血管扩张性共济失调突变蛋白(ATM)、磷酸化ATM(p-ATM)和抗γ-H2AX抗体购于美国Abcam公司,8-OHdG抗体购于美国Abbiotec公司。细胞培养箱(美国Billups-Rothenberg公司),荧光光谱仪(美国PerkinElmer公司),IX71倒置荧光相差显微镜(日本Olympus公司),Model550型全自动酶标仪(美国Bio-Rad公司)。
1.2 细胞培养HT22小鼠海马神经元细胞培养在DMEM培养液(含10%热灭活胎牛血清、1%谷氨酰胺、100 U·mL-1青霉素和100 mg·L-1链霉素)中,于37℃、95%O2和5%CO2的饱和湿度培养箱中培养,每3 d换液1次,待单层细胞达70%~80%融合后,采用0.25%胰酶消化后传代培养。
1.3 细胞分组和药物处理取对数生长期HT22小鼠海马神经元细胞种植于96孔培养板中,细胞密度为每孔5×103个,每组6孔。HT22细胞加入含10%胎牛血清的DMEM培养液中,在37℃、5%CO2条件下培养24 h后,弃去原培养液,加入含10%胎牛血清培养液2 h,将细胞随机分为空白对照组、2%七氟醚组(2%Sevo 6 h组、2%Sevo 12 h组和2%Sevo 24 h组)、4%七氟醚组(4%Sevo 6 h组、4%Sevo 12 h组和4%Sevo 24 h组)和8%七氟醚组(8%Sevo 6 h组、8%Sevo 12 h组和8%Sevo 24 h组)。采用MTT法和LDH法分别检测各组HT22小鼠海马神经元细胞存活率和死亡率。根据实验结果将细胞再次分为生理盐水组、生理盐水+4%Sevo 12 h组、生理盐水+8%Sevo 12 h组、NAC组、NAC+4%Sevo 12 h组和NAC+8%Sevo 12 h组,根据分组分别给予生理盐水或5 mmol·L-1NAC预处理1 h后,予以4%或8%浓度的七氟醚处理12 h。
1.4 MTT法检测细胞存活率将各组HT22小鼠海马神经元细胞进行相应的实验处理后接种于96孔培养板中,进行相应药物处理一定时间(6、12和24h)后,每孔加入20 μL MTT溶液,37℃孵育4 h后吸弃孔内上清液,每孔加入150 μL二甲基亚砜(DMSO),振荡10 min,采用酶标仪于490 nm波长处检测各孔吸光度(A)值,计算细胞存活率。细胞存活率=(实验组A值/对照组A值)×100%。
1.5 LDH法检测细胞死亡率将各组HT22小鼠海马神经元细胞进行相应的实验处理后接种于96孔培养板中,进行相应药物处理一定时间(6、12和24h)后,每孔加入20 μL LDH释放液,37℃避光孵育1 h后吸弃孔内上清液,离心5 min,取各自上清液120 μL转移至新的96孔培养板,加入LDH反应液60 μL,于25℃避光振荡30 min,采用酶标仪于490 nm波长处检测各孔A值,计算细胞死亡率。细胞死亡率=(实验组A值-对照组A值)/(空白对照组A值-对照组A值)×100%。
1.6 单细胞凝胶电泳(彗星实验)检测细胞DNA双链损伤程度参照BROZOVIC等[9]的实验方法,将第2次分组的HT22小鼠海马神经元细胞给予生理盐水或5 mmol·L-1NAC预处理1 h后,予以4%和8%七氟醚处理12 h,制备细胞悬液,用PBS缓冲液调整细胞密度为每毫升1×104~1×105个,采用低熔点琼脂糖溶解制片后经裂解、电泳、阅片,观察DNA迁移长度,以DNA迁移长度表示DNA双链损伤程度。
1.7 Western blotting法检测各组细胞中DNA损伤相关蛋白表达量向各组HT22海马神经元细胞中加入PBS缓冲液,进行3次离心后取上清,提取细胞总蛋白。采取BCA试剂盒进行蛋白定量。取各组蛋白质20~40 μg,经10%十二烷基硫酸钠(SDS)-聚丙烯酰胺凝胶电泳(PAGE)分离后,电转移至PVDF膜上。用5%脱脂奶粉封闭2h,分别加入1:500比例稀释的小鼠抗Histone、抗8-OHdG、抗ATM、抗p-ATM和抗γ-H2AX抗体,4℃孵育过夜,洗膜后加入辣根抗过氧化物酶标记的二抗,室温孵育2 h后洗膜,将PVDF膜用发光试剂ECL显色后成像。采用Image J凝胶图像分析软件分析蛋白条带灰度值,每个样本重复3次。
1.8 DCFH-DA染色法分析细胞中活性氧(ROS)水平将生理盐水组、生理盐水+4%Sevo 12 h组、生理盐水+8%Sevo 12 h组、NAC组、NAC+4%Sevo 12 h组和NAC+8%Sevo 12 h组HT22小鼠海马神经元细胞接种于96孔培养板中,给予生理盐水或NAC预处理1 h后,予以4%和8%七氟醚处理12 h,应用DCFH-DA染色,在激发波长502 nm、发射波长530 nm附近,使用荧光显微镜分析细胞中ROS荧光强度,以荧光强度表示ROS水平。
1.9 统计学分析采用SPSS 18.0统计软件对数据进行统计学分析。各组细胞存活率、细胞死亡率和细胞中ROS水平均符合正态分布,以x±s表示,多组间样本均数比较采用多因素方差分析,组间样本均数两两比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
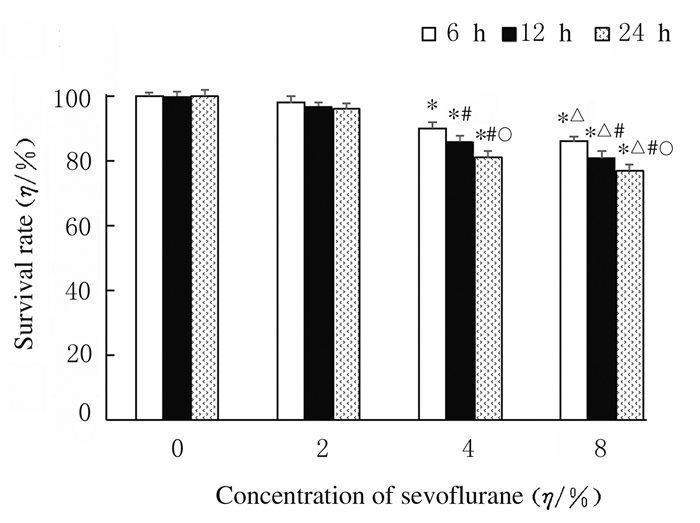
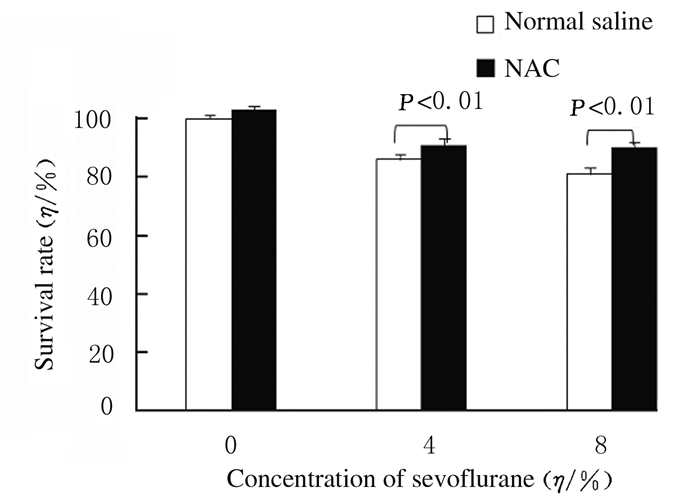
2 结果 2.1 各组HT22小鼠海马神经元细胞存活率与空白对照组比较,2%Sevo 6 h组、2%Sevo 12 h组和2%Sevo 24 h组细胞存活率差异无统计学意义(P>0.05);在同一浓度(4%Sevo组和8%Sevo组)时,七氟醚呈时间依赖性地抑制HT22小鼠海马神经元细胞存活(P < 0.05);与空白对照组比较,在同一时间点,4%和8%Sevo组HT22小鼠海马神经元细胞存活率明显降低(P < 0.01),且存活率随七氟醚浓度增加而降低(P < 0.05)。经NAC预处理后给予七氟醚,NAC+4% Sevo 12 h组和NAC+8%Sevo 12 h组细胞存活率分别为(91.3±2.1)%和(90.4±1.9)%,明显高于生理盐水+4%Sevo 12 h组(86.3%±1.7%)(P < 0.01)和生理盐水+8%Sevo 12 h组(81.4%±2.2%)(P < 0.01)。见图 1和图 2。
|
| *P < 0.01 compared with blank control group(0% sevoflurane group); △P < 0.05compared with 4%Sevo group; #P < 0.05 compared with 6 h; ○P < 0.05 compared with 12 h.. 图 1 MTT法检测各组HT22小鼠海马神经元细胞存活率 Fig. 1 Survival rates of HT22 mouse hippocampal neuronal cells of HT22 mice in various groups detected by MTT method |
|
|
|
| 图 2 MTT法检测NAC预处理后各组HT22小鼠海马神经元细胞存活率 Fig. 2 Survival rates of HT22 mouse hippocampal neuronal cells in various groups after NAC pretreatment detected by MTT method |
|
|
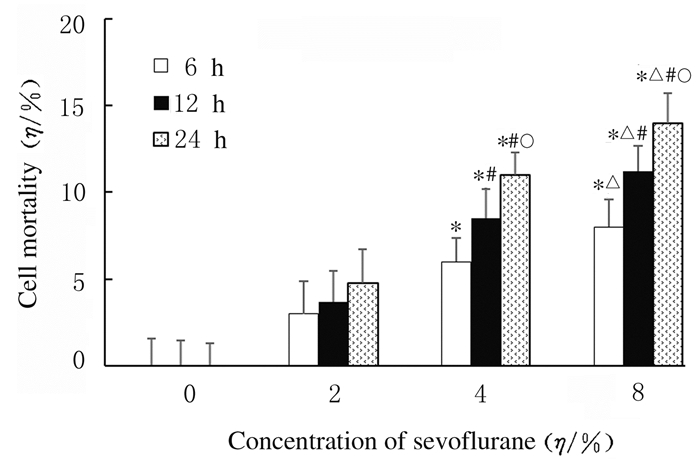
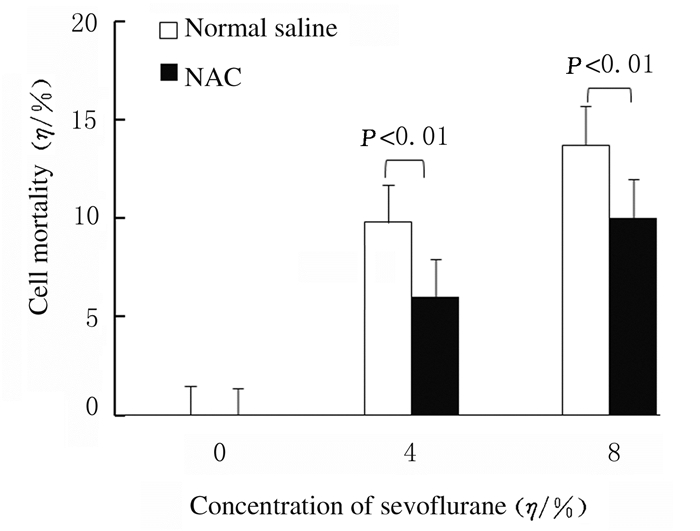
与空白对照组比较,2%Sevo 6 h组、2%Sevo 12 h组和2%Sevo 24 h组细胞死亡率差异无统计学意义(P>0.05);在同一浓度(4%Sevo组和8%Sevo组)时,七氟醚呈时间依赖性地促进HT22小鼠海马神经元细胞死亡(P < 0.05);与空白对照组比较,在同一时间点4%和8%Sevo组HT22小鼠海马神经元细胞死亡率明显升高(P < 0.01),且死亡率随着七氟醚浓度增加而升高(P < 0.05)。与生理盐水+4%Sevo 12 h组比较,NAC+4%Sevo 12 h组HT22小鼠海马神经元细胞死亡率明显降低(P < 0.01)。与生理盐水+8%Sevo 12 h组比较,NAC+8%Sevo 12 h组HT22小鼠海马神经元细胞死亡率明显降低(P < 0.01)。见图 3和图 4。
|
| * P < 0.01 compared with blank control group(0% sevoflurane group); △P < 0.05compared with 4% Sevo group; #P < 0.05 compared with 6 h; ○P < 0.05 compared with 12 h. 图 3 LDH法检测各组HT22小鼠海马神经元细胞死亡率 Fig. 3 Mortalities of HT22 mouse hippocampal neuronal cells in various groups detected by LDH assay |
|
|
|
| 图 4 LDH法检测NAC预处理后各组HT22小鼠海马神经元细胞死亡率 Fig. 4 Mortalities of HT22 mouse hippocampal neuronal cells in various groups after NAC pretreament detected by LDH assay |
|
|
与生理盐水组比较,生理盐水+4%Sevo 12 h组和生理盐水+8%Sevo 12 h组HT22小鼠海马神经元细胞DNA迁移长度明显增加,说明DNA双链损伤增多。与生理盐水+4%Sevo 12 h组比较,生理盐水+8%Sevo 12 h组DNA双链迁移长度增加。与生理盐水+4%Sevo 12 h组和生理盐水+8%Sevo 12 h组比较,NAC+4%Sevo 12 h组和NAC+8%Sevo 12h组DNA双链迁移长度明显减少,说明DNA损伤明显减少。见图 5(插页二)。

|
| A:Normal saline group; B:NAC group; C:Normal saline+4%Sevo12 h group; D: NAC+4%Sevo12 h group; E:Normal saline+ 8%Sevo12 h group; F:NAC+8%Sevo12 h group. 图 5 单细胞凝胶电泳检测各组HT22小鼠海马神经元细胞DNA双链损伤情况(×200) Fig. 5 DNA double-strand damage of HT22 mouse hippocampal neuronal cells in various groups detected by single-cell gel electrophoresis(×200) |
|
|
与空白对照组比较,2%Sevo 12 h组HT22小鼠海马神经元细胞中8-OHdG、ATM、p-ATM和γ-H2AX蛋白表达量无明显变化;与空白对照组和2%Sevo12 h组比较,4%Sevo 12 h组和8%Sevo 12 h组HT22小鼠海马神经元细胞中8-OHdG、ATM、p-ATM和γ-H2AX蛋白表达量明显增加。与生理盐水+4%Sevo 12h组和生理盐水+8%Sevo 12组比较,NAC+4%Sevo 12 h组和NAC+8%Sevo 12 h组,HT22小鼠海马神经元细胞中8-OHdG、ATM、p-ATM和γ-H2AX表达量均降低。见图 6和图 7。
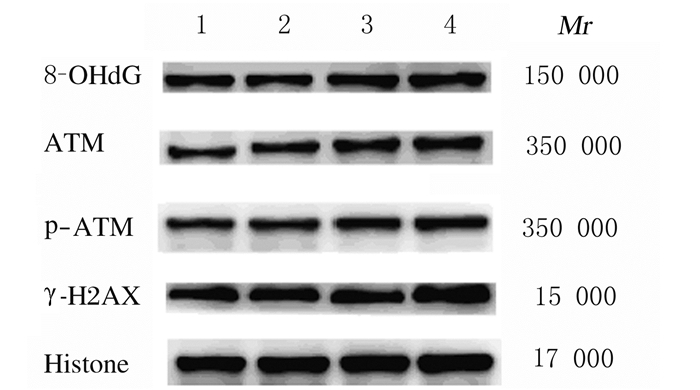
|
| Lane 1:Blank control group; Lane 2:2% Sevo 12 h group; Lane 3:4% Sevo 12 h group; Lane 4:8% Sevo 12 h group. 图 6 Western blotting法检测各组HT22小鼠海马神经元细胞中8-OHdG、ATM、p-ATM和γ-H2AX蛋白表达电泳图 Fig. 6 Electrophoregram of expressions of 8-OHdG, ATM, p-ATM and γ-H2AX proteins in HT22 mouse hippocampal neuronal cells in various groups detected by Western blotting method |
|
|

|
| Lane 1:Normal saline group; Lane 2:NAC group; Lane 3:Normal saline+4%Sevo 12 h grouop; Lane 4:NAC+4%Sevo 12 h group; Lane 5:Normal saline+8%Sevo 12 h group; Lane 6:NAC+8%Sevo 12 h group. 图 7 Western blotting法检测NAC预处理后各组HT22小鼠海马神经元细胞中8-OHdG、ATM、p-ATM和γ-H2AX蛋白表达电泳图 Fig. 7 Electrophoregram of expressions of 8-OHdG, ATM, p-ATM and γ-H2AX proteins in HT22 mouse hippocampal neuronal cells in various groups after NAC pretreatment detected by Western blotting method |
|
|
与生理盐水组比较,生理盐水+4%Sevo 12组和生理盐水8%Sevo12 h组可观察到HT22小鼠海马神经元细胞内有更明亮的绿色荧光,说明细胞内ROS积聚增多。与生理盐水+4%Sevo 12组和生理盐水+8%Sevo 12 h组比较,NAC+4%Sevo 12 h组和NAC+8%Sevo 12 h组HT22小鼠海马神经元细胞内绿色荧光明显减少,ROS水平明显降低(P < 0.01)。见图 8(插页二)和图 9。

|
| A:Normal saline group; B:NAC group; C:Normal saline+4%Sevo12 h group; D: NAC+4%Sevo12 h group; E:Normal saline+8%Sevo12 h group; F:NAC+8%Sevo12 h group. 图 8 荧光显微镜下各组HT22小鼠海马神经元细胞内ROS绿色荧光强度(×200) Fig. 8 ROS green fluorescence intensities in HT22 mouse hippocampal neuronal cells in various groups under fluorescence microscope(×200) |
|
|

|
| 图 9 各组HT22小鼠海马神经元细胞中ROS水平 Fig. 9 ROS levels in HT22 mouse hippocampal neuronal cells in various groups |
|
|
HT22小鼠海马神经元细胞是体外研究神经毒性的良好模型,广泛应用于脑神经性疾病研究中。临床上婴幼儿七氟醚最低肺泡有效浓度(MAC)值约为2.5%,这是本实验选择七氟醚用药浓度的依据。有研究[3-4, 10-12]显示:吸入麻醉药七氟醚能够引起不同种属的婴幼个体未成熟大脑产生广泛的神经毒性作用,导致神经元死亡。研究[13]表明:七氟醚能够抑制小鼠胚胎干细胞增殖分化,对胎儿发育产生毒性作用。本研究结果显示:临床相关浓度的七氟醚具有神经细胞毒性作用,能够时间和剂量依赖性地诱导HT22海马神经元细胞死亡、抑制细胞存活。本研究结果与上述观点相一致。
在生命进程中,DNA不断受到体内外多种有害因素刺激,如紫外线、毒性化学物质和细胞代谢过程中产生的氧自由基等,进而导致DNA损伤。DNA损伤可以表现多种类型,如DNA单链断裂、DNA双链断裂和DNA链交联等,这些受损DNA扰乱细胞稳态平衡,引起基因突变,导致细胞畸变、老化和死亡等。研究[14]表明:DNA损伤与多种神经系统疾病的发生相关,在这些神经系统疾病中存在DNA损伤及异常的DNA修复,最终导致神经元正常功能的缺失以及神经细胞死亡。单细胞凝胶电泳是检测细胞DNA双链损伤断裂的敏感方法。本研究检测了七氟醚对HT22小鼠海马神经元细胞DNA双链损伤的影响,结果发现:七氟醚能够诱导HT22小鼠海马神经元细胞DNA双链损伤,并与七氟醚的浓度呈正相关关系。为了进一步验证七氟醚对DNA损伤的影响,本研究检测了DNA损伤相关蛋白8-OHdG、ATM、p-ATM和γ-H2AX的表达。ATM是直接感受DNA双链断裂损伤并起始诸多DNA损伤信号反应通路的感受器,Ser1981的ATM磷酸化产生p-ATM,ATM磷酸化激活组蛋白H2AX产生磷酸化的γ-H2AX,进而启动DNA损伤修复过程。8-OHdG是活性氧自由基攻击DNA分子中的鸟嘌呤碱基第8位碳原子而产生的一种氧化性加和产物,其与DNA氧化损伤相关。本研究结果显示:与空白对照组比较,2%Sevo组HT22小鼠海马神经元细胞中DNA损伤相关蛋白8-OHdG、ATM、p-ATM和γ-H2AX表达量未发生明显改变。与空白对照组和2%Sevo组比较,4%Sevo组和8%Sevo组HT22小鼠海马神经元细胞中DNA损伤相关蛋白8-OHdG、ATM、p-ATM和γ-H2AX表达量明显增多,且与七氟醚的浓度有关联。NI等[8]研究证实吸入麻醉药异氟醚诱导老年小鼠大脑神经细胞死亡与DNA损伤密切相关。研究[15]发现:七氟醚和异氟醚暴露均能够造成青年志愿者外周血淋巴细胞中DNA损伤与断裂。
引起细胞内DNA损伤的原因大致可以分为环境因素、DNA自发性损伤以及细胞内产生过多的ROS等,其中细胞内的ROS增多是最引人关注的DNA损伤因素。ROS是细胞正常代谢过程中产生的副产物,生理情况下机体内ROS的生成系统与抗氧化清除系统之间保持动态平衡,病理状态下,二者之间平衡状态被破坏,细胞内ROS增多,引起氧化应激。ROS能够导致碱基发生突变,产生多种形式的DNA损伤,进而导致细胞死亡。大脑组织是人体所有组织器官中氧气消耗最多的部位,ROS的增加会引起明显的DNA氧化损伤和神经细胞死亡[12]。本研究结果显示:4%和8%七氟醚处理后,HT22小鼠海马神经元细胞中ROS水平升高,并与七氟醚的浓度呈依赖性。为进一步证实ROS与DNA损伤的相关性,预先给予抗氧化剂NAC后,HT22小鼠海马神经元细胞再给予七氟醚处理,结果发现:NAC能够明显抑制七氟醚诱导的HT22小鼠海马神经元细胞内ROS积聚、DNA损伤相关蛋白表达和DNA双链损伤,减少HT22小鼠海马神经元细胞死亡。本研究结果显示:七氟醚通过促进海马神经元细胞中ROS水平升高,导致DNA损伤相关蛋白表达增加,DNA双链损伤,进而导致海马神经元细胞死亡。研究[7, 16-22]显示:七氟醚能够诱导发育期的大脑还原型辅酶Ⅱ(NADPH)氧化酶和过氧化物酶的活化,抑制谷胱甘肽(GSH)和超氧化物歧化酶(SOD)的活性,促进ROS的生成。研究[16-22]显示:七氟醚暴露能够增强新生鼠脑氧化应激反应,损伤线粒体,诱导ROS生成和海马神经细胞死亡。
综上所述,七氟醚能够通过增加海马神经元细胞中ROS积聚,诱导DNA损伤,进而导致海马神经元细胞死亡。但本研究并未发现2%七氟醚具有明显的神经毒性作用,且基于七氟醚的诸多优点,不否认七氟醚的临床应用价值,仍需要进一步研究以确认七氟醚的神经毒性作用机制,以指导临床应用。
| [1] |
LIU YF, LIN D W, LIU C L, et al. Cyclin-dependent kinase 5/Collapsin response mediator protein 2 pathway may mediate sevoflurane-induced dendritic development abnormalities in rat cortical neurons[J]. Neurosci Lett, 2017, 651: 21-29. |
| [2] |
ANDROPOULOS D B. Effect of anesthesia on the developing brain:infant and fetus[J]. Fetal Diagn Ther, 2018, 43(1): 1-11. |
| [3] |
RAPER J, DE BIASIO J C, MURPHY K L, et al. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy[J]. Br J Anaesth, 2018, 120(4): 761-767. |
| [4] |
ALVARADO M C, MURPHY K L, BAXTER M G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy[J]. Br J Anaesth, 2017, 119(3): 517-523. |
| [5] |
LIU B, GU Y C, XIAO H Y, et al. Altered metabolomic profiles may be associated with sevoflurane-induced neurotoxicity in neonatal rats[J]. Neurochem Res, 2015, 40(4): 788-799. |
| [6] |
SUN Z, SATOMOTO M, ADACHI Y U, et al. Inhibiting NADPH oxidase protects against long-term memory impairment induced by neonatal sevoflurane exposure in mice[J]. Br J Anaesth, 2016, 117(1): 80-86. |
| [7] |
KISBY G E, MOORE H, SPENCER P S. Animal models of brain maldevelopment induced by cycad plant genotoxins[J]. Birth Defect Res C, 2013, 99(4): 247-255. |
| [8] |
NI C, LI C, DONG Y L, et al. Anesthetic isoflurane induces DNA damage through oxidative stress and p53 pathway[J]. Mol Neurobiol, 2017, 54(5): 3591-3605. |
| [9] |
BROZOVIC G, ORSOLIC N, ROZGAJ R, et al. Sevoflurane and isoflurane genotoxicity in kidney cells of mice[J]. Arch Ind Hyg Toxicol, 2017, 68(3): 228-235. |
| [10] |
ZHENG H, DONG Y L, XU Z P, et al. Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice[J]. Anesthesiology, 2013, 118(3): 516-526. |
| [11] |
PEREZ-ZOGHBI J F, ZHU W, GRAFE M R, et al. Dexmedetomidine-mediated neuroprotection against sevoflurane-induced neurotoxicity extends to several brain regions in neonatal rats[J]. Br J Anaesth, 2017, 119(3): 506-516. |
| [12] |
GENTRY K R, STEELE L M, SEDENSKY M M, et al. Early developmental exposure to volatile anesthetics causes behavioral defects in Caenorhabditis elegans[J]. Anesth Analg, 2013, 116(1): 185-189. |
| [13] |
YI X W, CAI Y R, ZHANG N, et al. Sevoflurane inhibits embryonic stem cell self-renewal and subsequent neural differentiation by modulating the let-7a-Lin28 signaling pathway[J]. Cell Tissue Res, 2016, 365(2): 319-330. |
| [14] |
ROSS C A, TRUANT R. DNA repair:A unifying mechanism in neurodegeneration[J]. Nature, 2017, 541(7635): 34-35. |
| [15] |
KARABIYIK L, SARDAC S, POLAT U, et al. Comparison of genotoxicity of sevoflurane and isoflurane in human lymphocytes studied in vivo using the comet assay[J]. Mutat Res/Genet Toxicol Environment Mutag, 2001, 492(1/2): 99-107. |
| [16] |
BHAT A H, DAR K B, ANEES S, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight[J]. Biomed Pharmacother, 2015, 74: 101-110. |
| [17] |
LV X, YAN J, JIANG J, et al. MicroRNA-27a-3p suppression of peroxisome proliferator-activated receptor-γ contributes to cognitive impairments resulting from sevoflurane treatment[J]. J Neurochem, 2017, 143(3): 306-319. |
| [18] |
ZHANG Y J, LI Y, HAN X C, et al. Elevated expression of DJ-1(encoded by the human PARK7 gene) protects neuronal cells from sevoflurane-induced neurotoxicity[J]. Cell Stress Chaperones, 2018, 23(5): 967-974. |
| [19] |
QI J L, JIA Y P, WANG W, et al. The role of BaG2 in neurotoxicity induced by the anesthetic sevoflurane[J]. J Cell Biochem, 2018, 120(5): 7551-7559. |
| [20] |
刘贝贝, 林晓婉, 郭航, 等. 孕酮对大鼠中枢神经系统发育期七氟醚神经毒性的作用[J]. 解放军医学杂志, 2019, 44(10): 831-836. |
| [21] |
YI W B, ZHANG Y, GUO Y M, et al. Elevation of Sestrin-2 expression attenuates Sevoflurane induced neurotoxicity[J]. Metab Brain Dis, 2015, 30(5): 1161-1166. |
| [22] |
XU G, LU H, DONG Y, et al. Coenzyme Q10 reduces sevoflurane-induced cognitive deficiency in young mice[J]. Br J Anaesth, 2017, 119(3): 481-491. |
 2020, Vol. 46
2020, Vol. 46


