扩展功能
文章信息
- 秦超, 戴曦, 杨小琼, 王荣丽, 王星, 李国平
- QIN Chao, DAI Xi, YANG Xiaoqiong, WANG Rongli, WANG Xing, LI Guoping
- 和厚朴酚对哮喘小鼠肺组织炎症反应的干预作用及其机制
- Intervention effect of honokiol on inflammatory response in lung tissue of asthma mice and its mechanism
- 吉林大学学报(医学版), 2020, 46(02): 214-220
- Journal of Jilin University (Medicine Edition), 2020, 46(02): 214-220
- 10.13481/j.1671-587x.20200202
-
文章历史
- 收稿日期: 2019-06-09
2. 西南医科大学附属医院炎症与变态反应实验室, 四川 泸州 646000;
3. 西南交通大学附属医院四川省成都市第三人民医院呼吸内科, 四川 成都 610000
2. Inflammation and Allergy Laboratory, Affiliated Hospital, Southwest Medical University, Luzhou 646000, China;
3. Department of Respiratory Medicine, Affiliated Hospital, Southwest Jiaotong University, Third People's Hospital of Chengdu City, Sichuan Province, Chengdu 610000, China
支气管哮喘(简称哮喘)是一种气道慢性炎症性疾病,其由多种细胞和细胞成分参与,发病机制极其复杂。近年来相关研究[1-2]表明:哮喘发病与氧化应激相关,机体内外环境的改变可导致氧化应激反应加剧,其对哮喘的病理生理过程有着重要的影响。在氧化应激反应中,活性氧(reactive oxygen and species, ROS)产生与抗氧化防御反应对抗的这一过程被认为与哮喘炎症的严重程度有关联。研究[3-4]显示:在哮喘患者中免疫应答过度表达可引发免疫细胞产生大量的活性氧和氮物质(reactive oxygen and nitrogen species, RONS),如羟基自由基、超氧化物、过氧化物、过氧亚硝酸盐和一氧化氮,RONS可以破坏气道上皮细胞的脂质和蛋白质,加重DNA的氧化损伤,进而导致哮喘病理生理过程趋于严重。目前临床治疗哮喘的主要方法是吸入皮质类固醇,但很大比例的哮喘患者无法从糖皮质激素治疗中获益,而约1/3的哮喘患者从白三烯抑制剂治疗中获益[5]。因此,哮喘的治疗目前仍是有待研讨的重要课题之一。和厚朴酚(honokiol, HNK)是从传统中药厚朴中提取纯化的主要有效治疗成分,现被证明具有抗氧化、抗炎、抗细菌和抗肿瘤药理作用[6]。目前关于HNK对哮喘治疗作用的研究还鲜有报道。本研究通过制备小鼠哮喘模型,探讨HNK对哮喘氧化应激及DNA损伤的抑制作用,从而探索治疗哮喘的潜在新药。
1 材料与方法 1.1 实验动物、主要试剂和仪器C57BL/6J健康雌性小鼠20只,购自重庆腾鑫生物有限公司,6~8周龄,体质量18~22g,动物合格证号:SCXK(京)2016-0002,饲养于西南医科大学动物实验中心SPF级动物房,自由进食及饮水。鸡卵清蛋白(ovalbumin,OVA)购自美国Sigma公司,氢氧化铝[Al(OH)3]购自成都市科龙化工试剂厂,HNK购自西安开来生物工程有限公司(批号:K188171),地塞米松(dexamethasone,DXM)购自上海通用药业股份有限公司(规格1 mL:5 mg,产品批号:1801233),小鼠白细胞介素4(interleukin-4,IL-4)、白细胞介素6(interleukin-6,IL-6)和白细胞介素17(interleukin-17,IL-17)检测试剂盒购自安迪华泰(中国)生物科技有限公司,超氧化物歧化酶(superoxide dismutase, SOD)、丙二醛(malondialdehyde, MDA)、谷胱甘肽过氧化物酶(glutathione peroxidase, GSH-Px)测定试剂盒购自南京建成生物工程研究所,核因子κB (nuclear factor-κB, NF-κB)、B淋巴细胞瘤2(Bcl-2)和含半胱氨酸的天冬氨酸蛋白水解酶3(Caspase-3)抗体购自艾博抗(上海)贸易有限公司,磷酸化的c-Jun氨基末端激酶(p-JNK)和H2AX组蛋白(γH2Ax)抗体购自美国CST公司。激光共聚焦荧光显微镜(德国Leica公司),Allegra X-22R低温离心机(美国Beckman Coulter公司),电子天平(德国Startorious公司),细胞计数板(美国Costar公司),化学发光成像系统(DM4000 B)(美国Bio-Rad公司),脱色摇床(北京六一仪器厂),低温恒温水浴槽(上海恒平科学仪器有限公司)。
1.2 实验动物分组、造模和给药将20只C57BL健康雌性小鼠随机分为对照组、模型组、DXM组和HNK组,每组5只。根据KIANMEHER等[7]的方法造模,模型组、DXM组和HNK组小鼠在实验第1、7和14天分别经腹腔注射OVA+Al(OH)3混悬液致敏;从第15天开始,OVA组、DXM组和HNK组小鼠在麻醉后经鼻滴入激发液OVA,连续激发7 d,同时DXM组和HNK组小鼠在每次激发前30 min分别腹腔注射DXM溶液2mg·kg-1及HNK溶液150 mg·kg-1,而对照组小鼠给予等量生理盐水。
1.3 各组小鼠肺组织病理学观察分离小鼠左肺组织,迅速放入4%多聚甲醛中固定,依次脱水、石蜡包埋、行HE染色,显微镜下观察小鼠肺组织炎症改变。
1.4 各组小鼠血清中IL-4、IL-6和IL-17水平检测于末次激发后24 h内麻醉小鼠,眼球采血,于4℃、以3 000 r·min-1离心15 min,收集血清,ELISA法检测小鼠血清中IL-4、IL-6和IL-17水平。
1.5 各组小鼠肺泡灌洗液细胞计数处死全部小鼠,结扎左肺,取1.5 mLPBS溶液行右肺泡灌洗并回收,回收率为85%以上,于4℃、1 000 r·min-1离心10 min,支气管肺泡灌洗液(bronchoalveolar lavage fluid,BALF),收集上清,沉淀细胞以1 mLPBS缓冲液重悬,行细胞总数计数,并涂片行快速迪夫染色后于光镜下行中性粒细胞和嗜酸性粒细胞计数。
1.6 各组小鼠肺组织匀浆中MDA水平和SOD及GSH-Px活性检测取少量右肺组织生理盐水中轻柔漂洗,滤纸吸干组织表面水分,电子秤上精确秤质量,加入9倍体积于肺组织的匀浆介质,置于冰面上手工匀浆,严格按照操作说明检测肺组织匀浆中MDA水平和SOD及GSH-Px活性。
1.7 Western blotting法检测各组小鼠肺组织中p-JNK、NF-κB、Caspase-3、Bcl-2和γH2Ax蛋白表达水平取右肺组织,加入适量液氮研磨成粉末状,加入1 mL裂解液冰上裂解30 min,于4℃、11 000 r·min-1离心10 min,BCA法测定样品蛋白浓度,确定上样量,依次电泳、转膜、封闭和抗体孵育,化学发光法检测,以蛋白条带的灰度值表示肺组织中p-JNK、NF-κB、Caspase-3、Bcl-2和γH2Ax蛋白表达水平。
1.8 各组小鼠肺组织中γH2Ax免疫荧光强度检测取出提前制备的肺组织冰冻切片,室温下放置,5%BSA溶液封闭10 min,加入约50 μL稀释的一抗覆盖,4℃过夜;PBS洗3次,加入50 μL相应荧光二抗,室温避光孵育1 h,PBS洗涤3次,加入50 μL DAPI染液,室温避光孵育5 min,PBS洗涤3次,封片,荧光显微镜下观察显像情况。
1.9 统计学分析采用SPSS 17.0统计软件进行统计学分析。各组小鼠炎性细胞总数,血清IL-4、IL-6和IL-17水平,肺组织匀浆中MDA水平和SOD及GSH-Px活性,肺组织中p-JNK、NF-κB、Caspase-3、Bcl-2和γH2Ax蛋白表达水平均符合正态分布,以x±s表示,多组间样本均数比较采用单因素方差分析,组间两两比较采用LSD法。以P < 0.05为差异有统计学意义。
2 结果 2.1 各组小鼠肺组织病理形态表现对照组小鼠肺组织无明显炎症改变;模型组小鼠可见支气管管壁、平滑肌层增厚,部分气道上皮细胞坏死脱落、支气管管腔狭窄,气道和血管周围可见大量的炎症细胞浸润;与模型组比较,DXM组和HNK组小鼠上述病理形态改变较轻,而HNK组小鼠上述病理形态表现与DXM组相似。见图 1(插页一)。
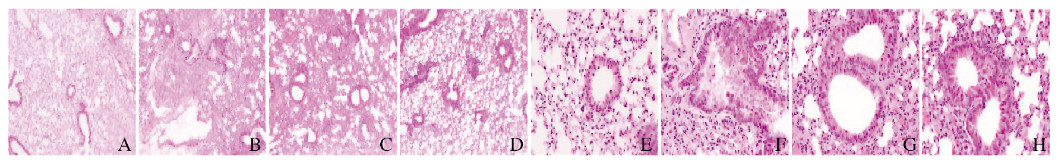
|
| A, E:Control group; B, F:Model group; C, G:DXM group; D, H:HNK group; A-D:×100;E-H:×400. 图 1 HE染色观察各组小鼠肺组织病理形态表现 Fig. 1 Pathomorphology of lung tissue of mice in various groups observed by HE staining |
|
|
与对照组比较,模型组、DXM组和HNK组小鼠BALF中炎性细胞总数、中性粒细胞总数和嗜酸性粒细胞总数明显增多(P < 0.05);与模型组比较,DXM组和HNK组小鼠BALF中炎性细胞总数、中性粒细胞总数和嗜酸性粒细胞总数减少(P < 0.05);HNK组小鼠BALF中炎性细胞浸润程度高于DXM组,但差异无统计学意义(P>0.05)。见表 1。
| (n=5, x±s) | |||||||||||||||||||||||||||||
| Group | Total number of inflammatorycells (×104mL-1) |
Total number of neutrophils (×103mL-1) |
Total number of eosinophils (×103mL-1) |
||||||||||||||||||||||||||
| Control | 9.69±2.40 | 0.25±0.07 | 0.68±0.23 | ||||||||||||||||||||||||||
| Model | 84.27±8.60* | 8.24±1.28* | 22.10±3.36* | ||||||||||||||||||||||||||
| DXM | 38.08±4.39*△ | 1.73±0.35*△ | 6.94±0.89*△ | ||||||||||||||||||||||||||
| HNK | 44.75±5.59*△ | 2.41±0.57*△ | 8.03±0.79*△ | ||||||||||||||||||||||||||
| F | 87.01 | 76.67 | 70.22 | ||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||||
| *P < 0.05 vs control group; △ P < 0.05 vs model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组、DXM组和HNK组小鼠血清中IL-4、IL-6和IL-17水平明显升高(P < 0.05);与模型组比较,DXM组和HNK组小鼠血清中IL-4、IL-6和IL-17水平降低(P < 0.05);而HNK组小鼠血清中IL-4、IL-6和IL-17水平高于DXM组,但差异无统计学意义(P>0.05)。见表 2。
| [n=5, x±s, ρB/(ng·L-1)] | |||||||||||||||||||||||||||||
| Group | IL-4 | IL-6 | IL-17 | ||||||||||||||||||||||||||
| Control | 9.16±0.66 | 4.87±0.91 | 8.22±1.15 | ||||||||||||||||||||||||||
| Model | 47.69±4.52* | 32.62±2.49* | 44.92±2.88* | ||||||||||||||||||||||||||
| DXM | 19.31±1.03*△ | 16.24±2.78*△ | 17.62±3.34*△ | ||||||||||||||||||||||||||
| HNK | 28.08±2.41*△ | 26.52±2.26*△ | 23.40±2.97*△ | ||||||||||||||||||||||||||
| F | 113.86 | 78.86 | 99.26 | ||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||||
| *P < 0.05 vs control group; △ P < 0.05 vs model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组、DXM组和HNK组小鼠肺组织匀浆中MDA水平明显升高(P < 0.05),而SOD和GSH-Px活性明显降低(P < 0.05);与模型组比较,DXM组和HNK组小鼠肺组织匀浆中MDA水平降低(P < 0.05),而SOD和GSH-Px活性升高(P < 0.05);HNK组小鼠MDA水平高于DXM组,SOD和GSH-Px活性低于DXM组,但差异均无统计学意义(P>0.05)。见表 3。
| (n=5, x±s) | |||||||||||||||||||||||||||||
| Group | MDA[cB/(mmol·L-1)] | SOD[λB/(U·mL-1)] | GSH-Px[λB/(U·mL-1)] | ||||||||||||||||||||||||||
| Control | 6.38±0.58 | 171.81±9.71 | 1 499.00±79.36 | ||||||||||||||||||||||||||
| Model | 17.30±1.81* | 77.85±10.48* | 890.69±57.54* | ||||||||||||||||||||||||||
| DXM | 11.07±0.97*△ | 132.62±12.30*△ | 1 284.26±35.51*△ | ||||||||||||||||||||||||||
| HNK | 9.66±0.58*△ | 94.90±12.09*△ | 1 107.98±144.35*△ | ||||||||||||||||||||||||||
| F | 51.22 | 36.46 | 16.716 | ||||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||||
| *P < 0.05 vs control group; △ P < 0.05 vs model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组、DXM组和HNK组小鼠肺组织中p-JNK、NF-κB、Caspase-3和γH2Ax蛋白表达水平明显升高(P < 0.05),模型组小鼠肺组织中Bcl-2蛋白表达水平明显降低(P < 0.05);与模型组比较,DXM组和HNK组小鼠肺组织中p-JNK、NF-κB、Caspase-3和γH2Ax蛋白表达水平降低(P < 0.05),Bcl-2蛋白表达水平升高(P < 0.05);HNK组小鼠肺组织中p-JNK、NF-κB、Caspase-3和γH2Ax蛋白表达水平高于DXM组,Bcl-2蛋白表达水平低于DXM组,但差异均无统计学意义(P>0.05)。见图 2和表 4。
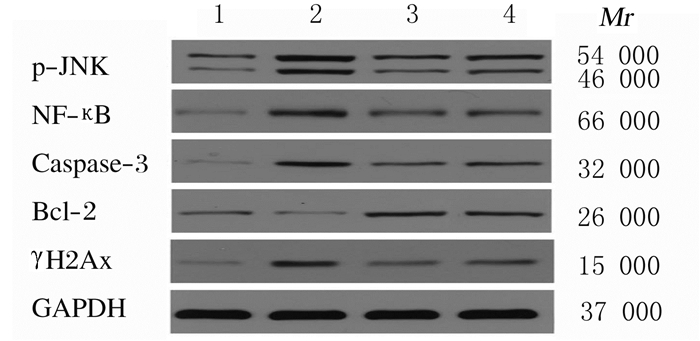
|
| Lane 1:Control group; Lane 2:Model group; Lane 3:DXM group; Lane 4:HNK group. 图 2 各组小鼠肺组织中p-JNK、NF-κB、Caspase-3、Bcl-2和γH2Ax蛋白表达电泳图 Fig. 2 Electrophoregram of expressions of p-JNK, NF-κB, Caspase-3, Bcl-2 and γH2Ax proteins in lung tissue of mice in various groups |
|
|
| (n=5, x±s) | |||||||||||||||||||||||||||||
| Group | p-JNK | NF-κB | Caspase-3 | Bcl-2 | γH2Ax | ||||||||||||||||||||||||
| Control | 0.39±0.04 | 0.10±0.08 | 0.03±0.01 | 0.13±0.02 | 0.06±0.02 | ||||||||||||||||||||||||
| Model | 1.25±0.06* | 0.62±0.06* | 0.46±0.05* | 0.07±0.02* | 0.46±0.10* | ||||||||||||||||||||||||
| DXM | 0.80±0.02*△ | 0.31±0.04*△ | 0.14±0.04*△ | 0.42±0.04*△ | 0.22±0.01*△ | ||||||||||||||||||||||||
| HNK | 0.87±0.02*△ | 0.36±0.04*△ | 0.19±0.07*△ | 0.39±0.03*△ | 0.27±0.02*△ | ||||||||||||||||||||||||
| F | 204.30 | 43.54 | 43.13 | 117.20 | 31.07 | ||||||||||||||||||||||||
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | ||||||||||||||||||||||||
| *P < 0.05 vs control group; △ P < 0.05 vs model group. | |||||||||||||||||||||||||||||
与对照组比较,模型组、DXM组和HNK组小鼠肺组织中γH2Ax荧光强度明显增强;与模型组比较,DXM组和HNK组小鼠肺组织中γH2Ax荧光强度有所减弱,而HNK组小鼠肺组织中γH2Ax免疫荧光强度与DXM组相似。见图 3(插页一)。
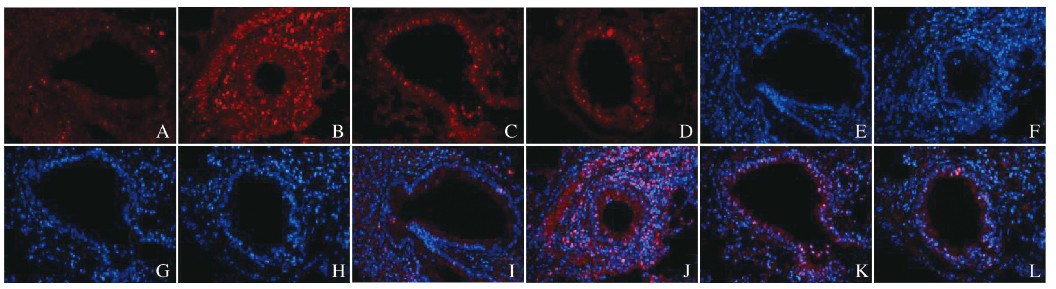
|
| A, E, I:Control group; B, F, J:Model group; C, G, K:DXM group; D, H, L:HNK group; A-D: γH2Ax protein; E-H:DAPI; I-L:Merge. 图 3 各组小鼠肺组织中γH2Ax免疫荧光强度(×200) Fig. 3 Immunofluorescence intensities of γH2AX in lung tissue of mice in various groups(×200) |
|
|
哮喘是一种慢性气道炎症性疾病,能够引起气道高反应性、可逆性气流受限、黏液高分泌和气道重塑[8]。哮喘发病机制复杂,其中Th1/Th2型细胞失衡是重要因素之一[9]。哮喘可加速Th2细胞活化,促进IL-4的产生,进而诱导B淋巴细胞活化分泌特异性IgE,后者可促进肥大细胞增殖分化,进一步刺激相关炎症细胞因子分泌,从而加重哮喘症状[10]。同时,单核细胞-巨噬细胞分泌的IL-6也可以促进Th2分化产生IL-4,进而加速哮喘的病理发展过程[11],而CD4+Th亚群中的Th17/Treg失衡是导致哮喘加重的另外因素之一。Th17活化分泌炎性因子IL-17,IL-17可促进支气管上皮纤维母细胞和平滑肌细胞增生,同时对中性粒细胞聚集浸润也发挥了重要作用[12-13],上述炎症因子的增多均可进一步加重哮喘症状。
研究[14]表明:哮喘小鼠气道中存在氧化应激水平升高以及DNA损伤现象。氧化应激反应可致抗氧化防御机制的失衡、ROS的大量产生,其中MDA作为脂质过氧化的最后产物,同时也是氧化应激的标志物,而GSH-Px和SOD则是保护细胞免受ROS损伤的重要抗氧化防御成分,其能清除体内的氧自由基,有效地防止ROS对机体细胞的损害[15]。此外,大量产生的ROS还可导致中性粒细胞浸润及多种形式的DNA损伤[16],激活MAPK和NF-κB信号通路,从而进一步诱导细胞凋亡[17-18]。MAPK信号通路可诱导哮喘中许多炎性介质的生成,其中p-JNK是介导细胞凋亡的重要调节靶点,其可被多种细胞因子和氧化损伤等激活[19-21]。同时,DNA损伤是细胞凋亡的启动因素也是必然结果,γH2Ax是反映DNA损伤程度和修复的生物标志物,其与细胞凋亡密切相关[22]。影响细胞凋亡的因素众多,Caspase-3蛋白酶作为细胞凋亡最为关键的因子之一,不仅可以反映细胞凋亡的程度,还可以参与细胞凋亡的过程,而Bcl-2家族则为调控细胞凋亡的家族,可阻断细胞凋亡的信号通路,从而抑制细胞凋亡的过程[23-24]。因此,通过药物干预哮喘可在一定程度上减轻气道上皮细胞凋亡及DNA损伤,从而控制哮喘病情。
HNK是从我国传统中药厚朴的干燥树皮或根皮中提取的一种天然多酚类化合物,现代药学研究[25]发现其具有抗炎、抗氧化、抗血管生成和抗肿瘤等药理作用。本研究结果显示:通过使用HNK干预哮喘小鼠可使小鼠坏死脱落的气道上皮细胞减少,在一定程度上减轻气道壁、气道平滑肌层增厚及管腔狭窄程度,还可减轻气道旁的炎性细胞浸润,表明HNK在治疗哮喘上有一定作用。同时,HNK组小鼠血清中炎性因子IL-4、IL-6和IL-17水平较模型组降低,提示HNK可适当减轻哮喘肺组织炎症反应。HNK组小鼠肺组织中MDA水平降低,而SOD和GSH-Px活性升高,说明HNK可以减轻氧化应激反应。进一步的研究结果显示:与模型组比较,HNK组小鼠肺组织中p-JNK、NF-κB、Caspase-3和γH2Ax蛋白表达水平降低,而Bcl-2蛋白表达水平升高,这表明HNK可能是通过抑制JNK和NF-κB介导的信号通路的信息转导,从而发挥减轻氧化应激反应、DNA损伤及减少气道上皮细胞凋亡的作用。
综上所述,HNK对哮喘小鼠肺组织炎症反应有一定的干预作用,其机制可能是通过抑制氧化应激反应及DNA损伤这一过程来实现的。本研究为HNK在哮喘临床治疗应用上提供了实验依据, 但该研究尚有不足之处,目前仅停留在动物实验阶段,且样本量较少,其具体的作用机制有待进一步探讨。
| [1] |
包爱华, 周新. 氧化应激及抗氧化治疗与支气管哮喘[J]. 中华哮喘杂志(电子版), 2012, 6(5): 359-365. DOI:10.3969/j.issn.1674-3911.2012.05.013 |
| [2] |
SAHINER U M, BIRBEN E, ERZURUM S, et al. Oxidative stress in asthma:Part of the puzzle[J]. Pediatr Allergy Immunol, 2018, 29(8): 789-800. DOI:10.1111/pai.12965 |
| [3] |
HO W E, CHENG C, PEH H Y, et al. Anti-malarial drug artesunate ameliorates oxidative lung damage in experimental allergic asthma[J]. Free Radic Biol Med, 2012, 53(3): 498-507. DOI:10.1016/j.freeradbiomed.2012.05.021 |
| [4] |
SLEIMAN PM, FLORY J, IMIELINSKI M, et al. Variants of DENND1B associated with asthma in children[J]. N Engl J Med, 2010, 362(1): 36-44. DOI:10.1056/NEJMoa0901867 |
| [5] |
MUNROE M E, BUSINGA T R, KLINE J N, et al. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma[J]. J Immunol, 2010, 185(9): 5586-5597. DOI:10.4049/jimmunol.1000630 |
| [6] |
WU F, YAO H P, ZHENG F P, et al. Protective effects of honokiol against oxidative stress induced apoptotic signaling in mouse podocytes treated with H2O2[J]. Exp Ther Med, 2018, 16(2): 1278-1284. |
| [7] |
KIANMEHER M, GHORANI V, BOSKABADY M H, et al. Animal model of asthma, various methods and measured parameters:a methodological review[J]. Iran J Allergy Asthma Immunol, 2016, 15(6): 445-465. |
| [8] |
KUDO M, ISHIGATSUBO Y, AOKI I. Pathology of asthma[J]. Front Microbiol, 2013, 4: 263. |
| [9] |
ZHU M, LIANG Z, WANG T, et al. Th1/Th2/Th17 cells imbalance in patients with asthma with and without psychological symptoms[J]. Allergy Asthma Proc, 2016, 37(2): 148-156. DOI:10.2500/aap.2016.37.3928 |
| [10] |
VAN RIJT L, VON RICHTHOFEN H, VAN REE R. Type 2 innate lymphoid cells:At the cross-roads in allergic asthma[J]. Semin Immunopathol, 2016, 38(4): 483-496. DOI:10.1007/s00281-016-0556-2 |
| [11] |
BHARTI R, DEY G, MANDAL M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells:a snapshot of IL-6 mediated involvement[J]. Cancer Lett, 2016, 375(1): 51-61. DOI:10.1016/j.canlet.2016.02.048 |
| [12] |
赵文娟, 袁嘉丽, 陈静, 等. 益气涤痰化瘀方对哮喘小鼠气道炎症及Th17/Treg平衡调节作用的研究[J]. 中华中医药学刊, 2015, 33(12): 2888-2891. |
| [13] |
MANNI M L, ROBINSON K M, ALCORN J F. A tale of two cytokines:IL-17 and IL-22 in asthma and infection[J]. Expert Rev Respir Med, 2014, 8(1): 25-42. DOI:10.1586/17476348.2014.854167 |
| [14] |
WANG Y F, LIN J T, SHU J, et al. Oxidative damage and DNA damage in lungs of an ovalbumin-induced asthmatic murine model[J]. J Thorac Dis, 2018, 10(8): 4819-4830. DOI:10.21037/jtd.2018.07.74 |
| [15] |
COLAKOGLU H E, YAZLIK M O, KAYA U, et al. MDA and GSH-Px activity in transition dairy cows under seasonal variations and their relationship with reproductive performance[J]. J Vet Res, 2017, 61(4): 497-502. DOI:10.1515/jvetres-2017-0067 |
| [16] |
CHAE W J, EHRLICH A K, CHAN P Y, et al. The wnt antagonist dickkopf-1 promotes pathological type 2 cell-mediated inflammation[J]. Immunity, 2016, 44(2): 246-258. DOI:10.1016/j.immuni.2016.01.008 |
| [17] |
GAO S, LI C J, CHEN L, et al. Actions and mechanisms of reactive oxygen species and antioxidative system in semen[J]. Mol Cell Toxicol, 2017, 13(2): 143-154. DOI:10.1007/s13273-017-0015-8 |
| [18] |
张一杨, 李莹淑, 李金凤, 等. SIRT1通过降低NF-κB p56表达减轻异烟肼致人肝细胞损伤的研究[J]. 中国现代医学杂志, 2018, 28(22): 7-12. DOI:10.3969/j.issn.1005-8982.2018.22.002 |
| [19] |
吴莎莎, 范晓云, 梁雅雪, 等. PLA激活JNK信号通路促进NCI-H292细胞凋亡[J]. 安徽医科大学学报, 2018, 53(6): 885-889. |
| [20] |
马望歌, 周栋, 王丽君, 等. IL-17A通过p38MAPK通路抑制巨噬细胞ABCA1蛋白的降解[J]. 西安交通大学学报(医学版), 2019, 40(6): 853-856. |
| [21] |
曹禹, 乔锐, 刘丹, 等. 趋化因子CCL2对血小板内p38MAPK-HSP27通路的活化作用[J]. 解放军医学杂志, 2018, 43(12): 1009-1012. DOI:10.11855/j.issn.0577-7402.2018.12.03 |
| [22] |
刘敏, 赵苒. γH2AX检测在DNA双链断裂研究中应用[J]. 中国公共卫生, 2015, 31(6): 742-746. |
| [23] |
PAZDRAK K, YOUNG T W, STAFFORD S, et al. Cross-talk between ICAM-1 and granulocyte-macrophage colony-stimulating factor receptor signaling modulates eosinophil survival and activation[J]. J Immunol, 2008, 180(6): 4182-4190. DOI:10.4049/jimmunol.180.6.4182 |
| [24] |
ZHENG W, MATEI N, PANG J W, et al. Delayed recanalization at 3 days after permanent MCAO attenuates neuronal apoptosis through FGF21/FGFR1/PI3K/Caspase-3 pathway in rats[J]. Exp Neurol, 2019, 320: 113007. DOI:10.1016/j.expneurol.2019.113007 |
| [25] |
HSIAO W L, LIU L. The role of traditional Chinese herbal medicines in cancer therapy-from TCM theory to mechanistic insights[J]. Planta Med, 2010, 76(11): 1118-1131. |
 2020, Vol. 46
2020, Vol. 46


